Label: VITRASE- hyaluronidase, ovine injection, solution
- NDC Code(s): 24208-002-02
- Packager: Bausch & Lomb Incorporated
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated February 8, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use VITRASE safely and effectively. See full prescribing information for VITRASE.
VITRASE ®(hyaluronidase injection), for infiltration use, for interstitial use, for intramuscular use, for intraocular use, for peribulbar use, for retrobulbar use, for soft tissue use, or for subcutaneous use
Initial U.S. Approval: 2004INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
- Draw the desired amount of VITRASE into the syringe to obtain target hyaluronidase activity (USP Units) according to table. ( 2.1)
- Before adding VITRASE to a solution containing another drug check appropriate references regarding physical/chemical incompatibilities. ( 7)
- Subcutaneous Fluid Administration: Inject 200 Units of VITRASE prior to clysis. It will facilitate absorption of 1,000 mL or more of solution. The dosage of subcutaneous fluids administered depends upon the age, weight, clinical condition of the patient, and laboratory determinations. Rate and volume of subcutaneous fluid administration should not exceed those employed for intravenous infusion. ( 2.2, 8.4)
- Increasing absorption and dispersion of injected drugs: Add 50–300 Units (most typically 150 Units) of VITRASE to the injection solution. ( 2.3)
- Subcutaneous urography: With the patient prone, inject 75 Units of VITRASE subcutaneously over each scapula, followed by injection of the contrast medium at the same sites. ( 2.4)
DOSAGE FORMS AND STRENGTHS
Injection: 200 USP units/mL in a single-dose vial ( 3)
CONTRAINDICATIONS
Hypersensitivity ( 4)
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
Allergic and anaphylactic-like reactions have been reported, rarely. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Bausch & Lomb Incorporated at 1-800-553-5340 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Furosemide, benzodiazepines and phenytoin are incompatible with hyaluronidase. ( 7.1)
- Hyaluronidase should not be used to enhance the absorption and dispersion of dopamine and/or alpha agonist drugs. ( 7.2)
- Local anesthetics: Hyaluronidase hastens onset and shortens duration of effect, increases incidence of systemic reactions. ( 7.3)
- Large doses of salicylates, cortisone, adrenocorticotropic hormone (ACTH), estrogens or antihistamines: Concomitant use may require larger amounts of hyaluronidase for equivalent dispersing effect. ( 7.4)
USE IN SPECIFIC POPULATIONS
Pediatric Use: The dosage of subcutaneous fluids administered is dependent upon the age, weight, and clinical condition of the patient. For premature infants or during the neonatal period, the daily dosage should not exceed 25 mL/kg of body weight, and the rate of administration should not be greater than 2 mL per minute. Special care must be taken in pediatric patients to avoid overhydration by controlling the rate and total volume of the infusion ( 2.2, 8.4).
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 1/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Subcutaneous Fluid Administration (Hypodermoclysis)
1.2 Dispersion and Absorption of Injected Drugs
1.3 Subcutaneous Urography
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
2.2 Dosage for Subcutaneous Fluid Administration (Hypodermoclysis)
2.3 Dosage for Absorption and Dispersion of Injected Drugs
2.4 Dosage for Subcutaneous Urography
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Spread of Localized Infection
5.2 Ocular Cornea Damage
5.3 Enzyme Inactivation with Intravenous Administration
6 ADVERSE REACTIONS
7 DRUG INTERACTIONS
7.1 Incompatibilities
7.2 Drug-Specific Precautions
7.3 Local Anesthetic Agent
7.4 Salicylates, Cortisone, ACTH, Estrogens, Antihistamines
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
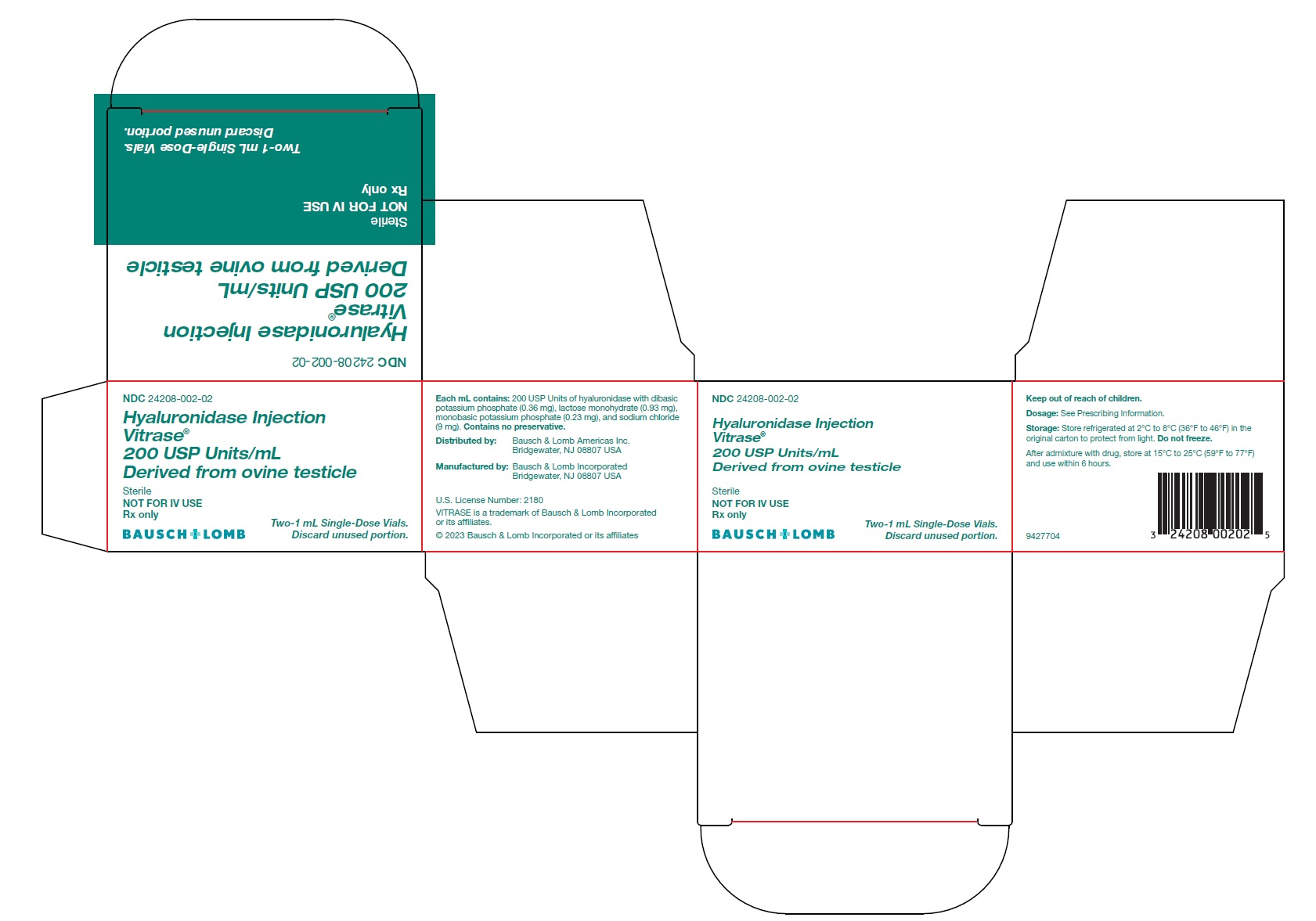
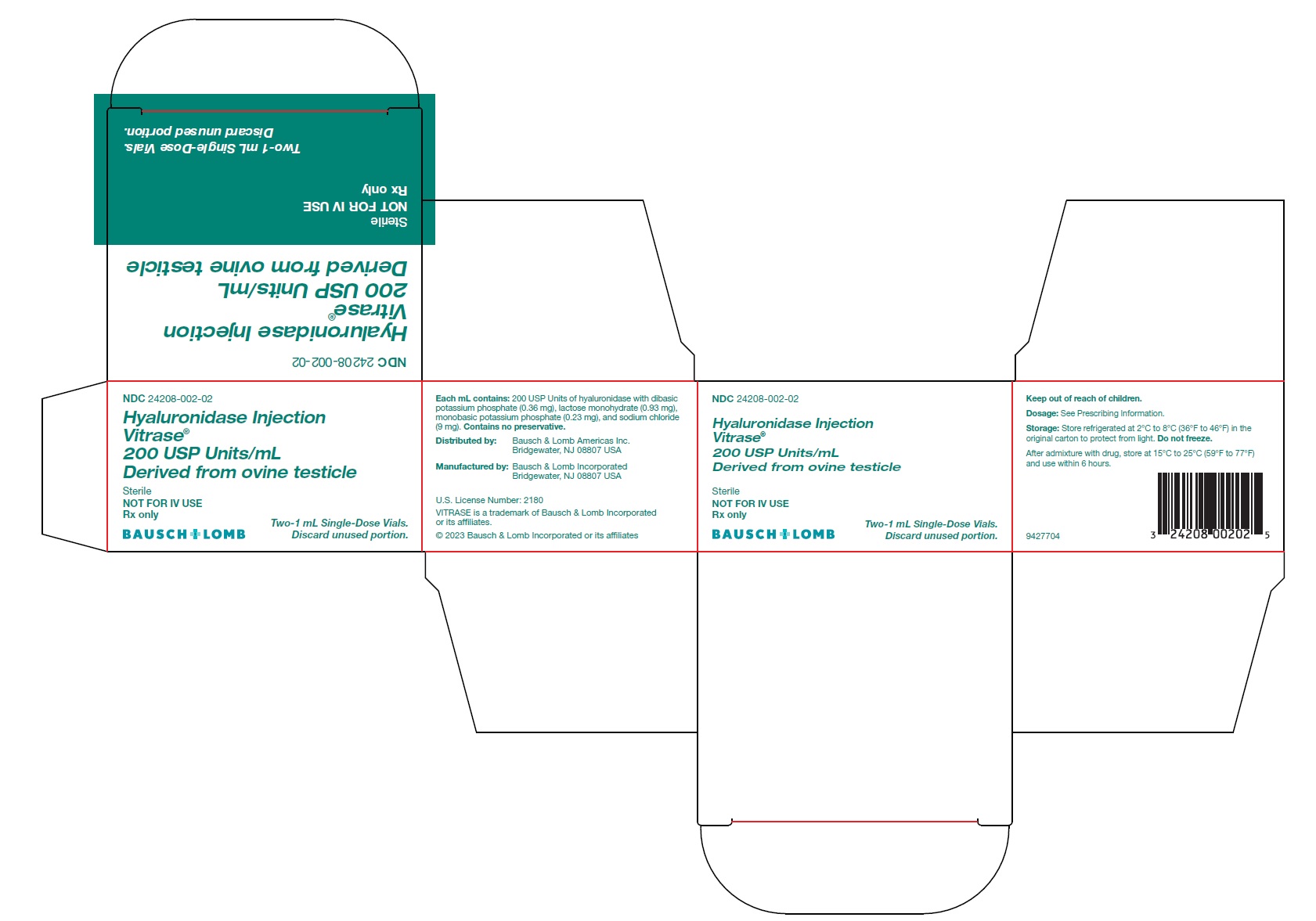
Hyaluronidase Injection
Vitrase ®
200 USP Units/mL
Derived from ovine testicle
Sterile
NOT FOR IV USE
Rx only- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 Subcutaneous Fluid Administration (Hypodermoclysis)
VITRASE ®(hyaluronidase injection) is indicated as an adjuvant in subcutaneous fluid administration for achieving hydration.
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
VITRASE should be administered as discussed below, since its effects relative to absorption and dispersion of other drugs are not produced when it is administered intravenously.
Draw the desired amount of VITRASE into the syringe to obtain the target Hyaluronidase Activity (USP Units) according to the table below.
Table 1. Amount of VITRASE Solution
Withdrawn Per Target
Hyaluronidase Activity
Target Hyaluronidase
Activity (USP Units)
Volume
Withdrawn
from Vial (mL)50 Units
0.25 mL
75 Units
0.38 mL
150 Units
0.75 mL
200 Units
1.00 mL
After admixture with drug, store at 15°C to 25°C (59°F to 77°F) and use within 6 hours. Consult the Prescribing Information of other drugs for additional storage information.
2.2 Dosage for Subcutaneous Fluid Administration (Hypodermoclysis)
Insert needle with aseptic precautions. With tip free and movable between skin and muscle, begin clysis; fluid should start in readily without pain or lump. Then inject VITRASE into rubber tubing close to needle.
An alternate method is to inject VITRASE under skin prior to clysis. 200 Units will facilitate absorption of 1,000 mL or more of solution. As with all parenteral fluid therapy, observe effect closely, with same precautions for restoring fluid and electrolyte balance as in intravenous injections. The dose, the rate of injection, and the type of solution (saline, glucose, Ringer’s, etc.) must be adjusted carefully to the individual patient. When solutions devoid of inorganic electrolytes are given by hypodermoclysis, hypovolemia may occur. This may be prevented by using solutions containing adequate amounts of inorganic electrolytes and/or controlling the volume and speed of administration.
VITRASE may be added to small volumes of solution (up to 200 mL), such as small clysis for infants or solutions of drugs for subcutaneous injection. For infants and children less than 3 years old, the volume of a single clysis should be limited to 200 mL; and in premature infants or during the neonatal period, the daily dosage should not exceed 25 mL/kg of body weight; the rate of administration should not be greater than 2 mL per minute. For older patients, the rate and volume of administration should not exceed those employed for intravenous infusion.
2.3 Dosage for Absorption and Dispersion of Injected Drugs
Absorption and dispersion of other injected drugs may be enhanced by adding 50 Units to 300 Units, most typically 150 Units of VITRASE to the injection solution.
2.4 Dosage for Subcutaneous Urography
The subcutaneous route of administration of urographic contrast media is indicated when intravenous administration cannot be successfully accomplished, particularly in infants and small children. With the patient prone, 75 Units of VITRASE are injected subcutaneously over each scapula, followed by injection of the contrast medium at the same sites.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
-
6 ADVERSE REACTIONS
The following adverse reactions have been identified during post-approval use of hyaluronidase products. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. The most frequently reported adverse reactions have been local injection site reactions.Hyaluronidase has been reported to enhance the adverse reactions associated with co-administered drug products. Edema has been reported most frequently in association with hypodermoclysis.
Allergic reactions (e.g., urticaria, angioedema) have been reported in less than 0.1% of patients receiving hyaluronidase. Anaphylactic-like reactions following retrobulbar block or intravenous injections have occurred, rarely.
-
7 DRUG INTERACTIONS
It is recommended that appropriate references be consulted regarding physical or chemical incompatibilities before adding VITRASE to a solution containing another drug.
7.1 Incompatibilities
Furosemide, benzodiazepines and phenytoin have been found to be incompatible with hyaluronidase.
7.2 Drug-Specific Precautions
Hyaluronidase should not be used to enhance the absorption and dispersion of dopamine and/or alpha agonist drugs.
When considering the administration of any other drug with hyaluronidase, it is recommended that appropriate references first be consulted to determine the usual precautions for the use of the other drug.
7.3 Local Anesthetic Agent
When hyaluronidase is added to a local anesthetic agent, it hastens the onset of analgesia and tends to reduce the swelling caused by local infiltration, but the wider spread of the local anesthetic solution increases its absorption; this shortens its duration of action and tends to increase the incidences of systemic reaction.
7.4 Salicylates, Cortisone, ACTH, Estrogens, Antihistamines
Patients receiving large doses of salicylates, cortisone, adrenocorticotropic hormone (ACTH), estrogens, or antihistamines may require larger amounts of hyaluronidase for equivalent dispersing effect, since these drugs apparently render tissues partly resistant to the action of hyaluronidase.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Human studies of hyaluronidase as an aid to conception and as an aid to delivery have been conducted without reports of maternal or fetal harm. Non-human animal reproduction studies have not been conducted with VITRASE.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. However, the background risk in the U.S. general population of major birth defects is 2 to 4%, and of miscarriage is 15 to 20%, of clinically recognized pregnancies.
Clinical Considerations
Hyaluronidase has been used as a component to aid the in vitrofertilization of human eggs. Administration of hyaluronidase during labor was reported to cause no complications; no increase in blood loss or differences in cervical trauma were observed.
8.2 Lactation
Risk Summary
There is no information regarding the presence of VITRASE in human milk, the effects on breastfed infants, or the effects on milk production to inform risk of VITRASE to an infant during lactation. The developmental and health benefits of breastfeeding should be considered, along with the mother’s clinical need for VITRASE, and any potential adverse effects on the breastfed infant from VITRASE.
8.4 Pediatric Use
The safety and effectiveness of VITRASE have been established in pediatric patients. Use of VITRASE in these patients is supported by evidence from adequate and well-controlled studies. Clinical hydration requirements for children can be achieved through administration of subcutaneous fluids facilitated with VITRASE.
The dosage of subcutaneous fluids administered is dependent upon the age, weight, and clinical condition of the patient as well as laboratory determinations. The potential for chemical or physical incompatibilities should be kept in mind [ see Drug Interactions (7)] .
The rate and volume of subcutaneous fluid administration should not exceed those employed for intravenous infusion. For premature infants or during the neonatal period, the daily dosage should not exceed 25 mL/kg of body weight, and the rate of administration should not be greater than 2 mL per minute.
During subcutaneous fluid administration, special care must be taken in pediatric patients to avoid overhydration by controlling the rate and total volume of the infusion [see Dosage and Administration (2.2)] .
-
11 DESCRIPTION
Hyaluronidase is an endoglycosidase. VITRASE is a preparation of purified ovine testicular hyaluronidase, a protein enzyme. Hyaluronidase is composed of two major glycosylated forms, α and β. The exact chemical structure of this enzyme is unknown.
VITRASE (hyaluronidase injection) is supplied as a sterile, non-preserved, clear and colorless 1 mL solution in a single-dose vial for infiltration use, for interstitial use, for intramuscular use, for intraocular use, for peribulbar use, for retrobulbar use, for soft tissue use, or for subcutaneous use.
Each mL contains 200 USP units of hyaluronidase with dibasic potassium phosphate (0.36 mg), lactose monohydrate (0.93 mg), monobasic potassium phosphate (0.23 mg), and sodium chloride (9 mg). The solution has a pH of 6.4 to 7.2.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Hyaluronidase is a spreading or diffusing substance, which modifies the permeability of connective tissue through the hydrolysis of hyaluronic acid, a polysaccharide found in the intercellular ground substance of connective tissue, and of certain specialized tissues, such as the umbilical cord and vitreous humor. Hyaluronic acid is also present in the capsules of type A and C hemolytic streptococci. Hyaluronidase hydrolyzes hyaluronic acid by splitting the glucosaminidic bond between C1 of the glucosamine moiety and C4 of glucuronic acid. This temporarily decreases the viscosity of the cellular cement and promotes diffusion of injected fluids or of localized transudates or exudates, thus facilitating their absorption.
Hyaluronidase cleaves glycosidic bonds of hyaluronic acid and, to a variable degree, some other acid mucopolysaccharides of the connective tissue. The activity is measured in vitroby monitoring the decrease in the amount of an insoluble serum albumen-hyaluronic acid complex as the enzyme cleaves the hyaluronic acid component.
12.2 Pharmacodynamics
In the absence of hyaluronidase, material injected subcutaneously spreads very slowly. Hyaluronidase facilitates dispersion, provided local interstitial pressure is adequate to furnish the necessary mechanical impulse. Such an impulse is normally initiated by injected solutions. The rate and extent of dispersion and absorption is proportionate to the amount of hyaluronidase and the volume of solution.
The reconstitution of the dermal barrier removed by intradermal injection of hyaluronidase (over a range of 0.002 to 20 Units/mL) to adult humans indicated that at 24 hours the restoration of the barrier is incomplete and inversely related to the dosage of enzyme; at 48 hours the barrier is completely restored in all treated areas.
Results from an experimental study, in humans, on the influence of hyaluronidase in bone repair support the conclusion that this enzyme alone, in the usual clinical dosage, does not deter bone healing.
12.3 Pharmacokinetics
Knowledge of the mechanisms involved in the disappearance of injected hyaluronidase is limited. It is known, however, that the blood of a number of mammalian species including humans brings about the inactivation of hyaluronidase.
Studies have demonstrated that hyaluronidase is antigenic; repeated injections of relatively large amounts of this enzyme may result in the formation of neutralizing antibodies.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Animal studies have not been conducted to determine whether hyaluronidase has carcinogenic or mutagenic potential.
Adequate fertility studies have not been conducted in animals, however, it has been reported that testicular degeneration may occur from the production of organ-specific antibodies against this enzyme following repeated injections.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
VITRASE ®(hyaluronidase injection) is supplied as 200 USP units/mL of sterile, non-preserved, clear and colorless solution in a single-dose, glass vial with a rubber stopper and aluminum seal. Discard unused portion.
- NDC 24208-002-02: 200 USP units/mL in a single-dose vial (NDC 24208-002-03) available in a carton containing 2 single-dose vials.
16.2 Storage and Handling
- Protect from light.
- Store unopened vial in refrigerator at 2°C to 8°C (36°F to 46°F).
- Do not freeze.
-
17 PATIENT COUNSELING INFORMATION
Reporting Adverse Reactions
Instruct patients to report adverse reactions including redness, swelling, itching, or pain at the injection site.
Interactions with Other Medications
Instruct patients to report if they are taking furosemide, benzodiazepines, phenytoin, dopamine and/or alpha agonists because these medications have been found to be incompatible with hyaluronidase.
Instruct patients to report if they are taking salicylates (e.g., aspirin), steroids (e.g., cortisone or estrogens) or antihistamines because larger amounts of hyaluronidase may be needed to achieve an equivalent dispersing effect.
Distributed by:
Bausch & Lomb Americas Inc.
Bridgewater, NJ 08807 USA
Manufactured by:
Bausch & Lomb Incorporated
Bridgewater, NJ 08807 USA
U.S. License Number: 2180
VITRASE is a trademark of Bausch & Lomb Incorporated or its affiliates.© 2023 Bausch & Lomb Incorporated or its affiliates
9427904
- PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – Vitrase Carton Label
-
INGREDIENTS AND APPEARANCE
VITRASE
hyaluronidase, ovine injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:24208-002 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYALURONIDASE (OVINE) (UNII: 64R4OHP8T0) (HYALURONIDASE, OVINE - UNII:64R4OHP8T0) HYALURONIDASE (OVINE) 200 [USP'U] in 1 mL Inactive Ingredients Ingredient Name Strength MONOBASIC POTASSIUM PHOSPHATE (UNII: 4J9FJ0HL51) 0.23 mg in 1 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) 9 mg in 1 mL LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) DIBASIC POTASSIUM PHOSPHATE (UNII: CI71S98N1Z) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:24208-002-02 2 in 1 CARTON 02/01/2005 1 1.2 mL in 1 VIAL, SINGLE-USE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA021640 02/01/2005 Labeler - Bausch & Lomb Incorporated (196603781) Establishment Name Address ID/FEI Business Operations Alliance Medical Products, Inc. (dba Siegfried Irvine) 102688657 manufacture(24208-002)