NASAL DECONGESTANT MAXIMUM STRENGTH- pseudoephedrine hcl tablet
SUPERVALU INC.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Equaline 44-112-Delisted
Uses
- temporarily relieves nasal congestion due to the common cold, hay fever or other upper respiratory allergies
- temporarily relieves sinus congestion and pressure
Warnings
Do not use
if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- thyroid disease
- diabetes
- heart disease
- high blood pressure
- trouble urinating due to an enlarged prostate gland
Directions
|
adults and children | take 2 tablets every 4 to 6 hours; do not take more than 8 tablets in 24 hours |
| children ages 6 to 12 years | take 1 tablet every 4 to 6 hours; do not take more than 4 tablets in 24 hours |
| children under 6 years | do not use this product in children under 6 years of age |
Other information
- store at 25°C (77°F); excursions permitted between 15°-30°C (59°-86°F)
- see end flap for expiration date and lot number
Inactive ingredients
croscarmellose sodium, dicalcium phosphate, FD&C red #40 aluminum lake, FD&C yellow #6 aluminum lake, hypromellose, magnesium stearate, microcrystalline cellulose, polydextrose, polyethylene glycol, silica gel, titanium dioxide, triacetin
Principal Display Panel
EQUALINE®
NDC 41163-112-08
compare to
Sudafed® Congestion
active ingredient*
maximum strength
nasal decongestant
pseudoephedrine hydrochloride tablets, 30 mg
(nasal decongestant)
non-drowsy
pseudoephedrine HCl
relieves:
• nasal & sinus congestion
• sinus pressure
24 tablets, 30mg each
actual size
TAMPER EVIDENT: DO NOT USE IF
CARTON IS OPENED OR IF BLISTER
UNIT IS TORN, BROKEN OR SHOWS
ANY SIGNS OF TAMPERING
SUPERVALU QUALITY
Guaranteed
We’re committed to your
satisfaction and guarantee
the quality of this product.
Contact us at 1-877-932-7948, or
www.supervalu-ourownbrands.com.
Please have package available.
DOES NOT CONTAIN GLUTEN
*This product is not manufactured or distributed by McNeil
Consumer Healthcare, owner of the registered trademark
Sudafed® Congestion. 50844 REV0712B11208
DISTRIBUTED BY SUPERVALU INC.
EDEN PRAIRIE, MN 55344 USA
Equaline 44-112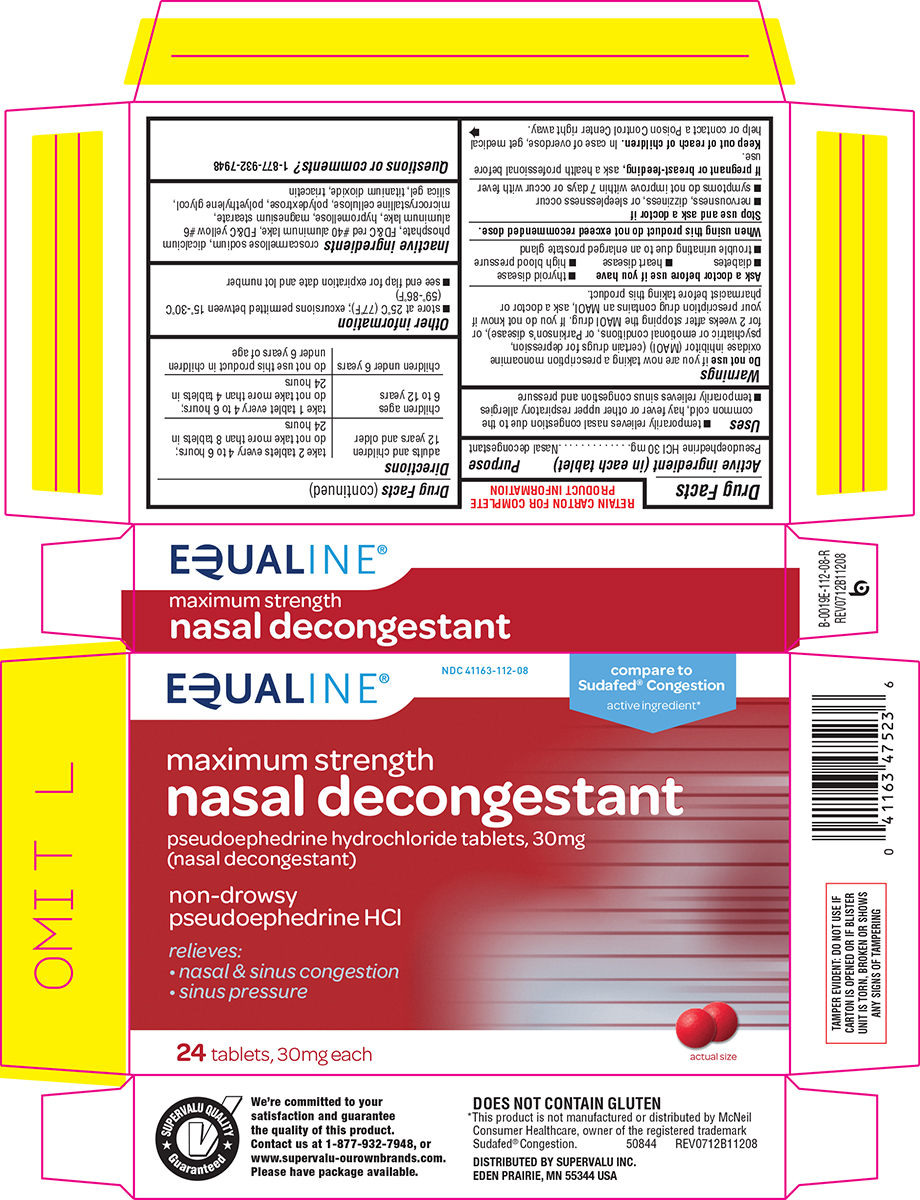
| NASAL DECONGESTANT
MAXIMUM STRENGTH
pseudoephedrine hcl tablet |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Labeler - SUPERVALU INC. (006961411) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 832867894 | manufacture(41163-112) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 038154464 | pack(41163-112) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 967626305 | pack(41163-112) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 832867837 | pack(41163-112) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 868734088 | pack(41163-112) | |