Label: TERCONAZOLE suppository
- NDC Code(s): 45802-717-00, 45802-717-08
- Packager: Padagis Israel Pharmaceuticals Ltd
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 3, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
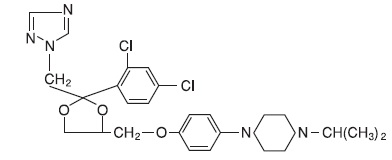
Terconazole Vaginal Suppositories, 80 mg are white to off-white suppositories for intravaginal administration containing 80 mg of the antifungal agent terconazole, cis-1-[p-[[2-(2,4-Dichlorophenyl)-2- (1H-1,2,4-triazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy]phenyl]-4- isopropylpiperazine, in triglycerides derived from coconut and/or palm kernel oil (a base of hydrogenated vegetable oils) and butylated hydroxyanisole.
The structural formula of terconazole is as follows:
Terconazole, a triazole derivative, is a white to almost white powder with a molecular weight of 532.47. It is insoluble in water; sparingly soluble in ethanol; and soluble in butanol.
-
CLINICAL PHARMACOLOGY
Absorption - Following a single intravaginal application of a suppository containing 240 mg 14C-terconazole to healthy women, approximately 70% (range: 64-76%) of terconazole remains in the vaginal area during the suppository retention period (16 hours); approximately 10% (range: 5-16%) of the administered radioactivity was absorbed systemically over 7 days. Maximum plasma concentrations of terconazole occur 5 to 10 hours after intravaginal application of the suppository. Systemic exposure to terconazole is approximately proportional to the applied dose. The rate and extent of absorption of terconazole are similar in patients with vulvovaginal candidiasis (pregnant or non-pregnant) and healthy subjects.
Distribution - Terconazole is highly protein bound (94.9%) in human plasma and the degree of binding is independent of drug concentration over the range of 0.01 to 5.0 mcg/mL.
Metabolism - Systemically absorbed terconazole is extensively metabolized (>95%).
Elimination - Across various studies in healthy women, after single or multiple intravaginal administration of terconazole, the mean elimination half-life of unchanged terconazole ranged from 6.4 to 8.5 hours. Following a single intravaginal administration of a suppository containing 240 mg 14C-terconazole to hysterectomized or tubal ligated women, approximately 3 to 10% (mean ± SD: 5.7 ± 3.0%) of the administered radioactivity was eliminated in the urine and 2 to 6% (mean ± SD: 4.2 ± 1.6%) was eliminated in the feces during the 7-day collection period.
Multiple Dosing - There is no significant increase in maximum plasma concentration or overall exposure (AUC) after multiple daily applications of the suppositories for 3 days.
Photosensitivity reactions have not been observed in U.S. and foreign clinical trials in patients who were treated with terconazole suppositories.
Microbiology
Mechanism of action - Terconazole, an azole antifungal agent, inhibits fungal cytochrome P-450-mediated 14 alpha-lanosterol demethylase enzyme. This enzyme functions to convert lanosterol to ergosterol. The accumulation of 14 alpha-methyl sterols correlates with the subsequent loss of ergosterol in the fungal cell wall and may be responsible for the antifungal activity of terconazole. Mammalian cell demethylation is less sensitive to terconazole inhibition.
Activity in vitro - Terconazole exhibits antifungal activity in vitro against Candida albicans and other Candida species. The MIC values of terconazole against most Lactobacillus spp. typically found in the human vagina were ≥128 mcg/mL; therefore these beneficial bacteria are not affected by drug treatment.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
- WARNINGS
-
PRECAUTIONS
General -
For vulvovaginal use only. Terconazole Vaginal Suppositories, 80 mg is not for ophthalmic or oral use. Discontinue use and do not retreat with terconazole if sensitization, irritation, fever, chills or flu-like symptoms are reported during use.
The base contained in the suppository formulation may interact with certain rubber or latex products, such as those used in vaginal contraceptive diaphragms or latex condoms; therefore concurrent use is not recommended.
Laboratory Tests -
If there is a lack of response to terconazole, appropriate microbiologic studies (standard KOH smear and/or cultures) should be repeated to confirm the diagnosis and rule out other pathogens.
Drug Interactions -
The therapeutic effect of terconazole is not affected by oral contraceptive usage.
The levels of estradiol and progesterone did not differ significantly when 0.8% terconazole vaginal cream was administered to healthy female volunteers established on a low dose oral contraceptive.
Carcinogenesis -
Studies to determine the carcinogenic potential of terconazole have not been performed.
Mutagenicity -
Terconazole was not mutagenic when tested in vitro for induction of microbial point mutations (Ames test), or for inducing cellular transformation, or in vivo for chromosome breaks (micronucleus test) or dominant lethal mutations in mouse germ cells.
Impairment of Fertility -
No impairment of fertility occurred when female rats were administered terconazole orally up to 40 mg/kg/day for a three month period.
Pregnancy: Teratogenic Effects: Pregnancy Category C -
There was no evidence of teratogenicity when terconazole was administered orally up to 40 mg/kg/day (25x the recommended intravaginal human dose of the suppository formulation) in rats, or 20 mg/kg/day in rabbits, or subcutaneously up to 20 mg/kg/day in rats.
Dosages at or below 10 mg/kg/day produced no embryotoxicity; however, there was a delay in fetal ossification at 10 mg/kg/day in rats. There was some evidence of embryotoxicity in rabbits and rats at 20-40 mg/kg. In rats, this was reflected as a decrease in litter size and number of viable young and reduced fetal weight. There was also delay in ossification and an increased incidence of skeletal variants.
The no-effect dose of 10 mg/kg/day resulted in a mean peak plasma level of terconazole in pregnant rats of 0.176 mcg/mL which exceeds by 17 times the mean peak plasma level (0.010 mcg/mL) seen in normal subjects after intravaginal administration of terconazole 80 mg vaginal suppository. This safety assessment does not account for possible exposure of the fetus through direct transfer to terconazole from the irritated vagina by diffusion across amniotic membranes. Since terconazole is absorbed from the human vagina, it should not be used in the first trimester of pregnancy unless the physician considers it essential to the welfare of the patient.
Terconazole may be used during the second and third trimester if the potential benefit outweighs the possible risks to the fetus.
Nursing Mothers -
It is not known whether this drug is excreted in human milk. Animal studies have shown that rat offspring exposed via the milk of treated (40 mg/kg/orally) dams showed decreased survival during the first few post-partum days, but overall pup weight and weight gain were comparable to or greater than controls throughout lactation. Because many drugs are excreted in human milk, and because of the potential for adverse reaction in nursing infants from terconazole, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Geriatric Use -
Clinical studies of terconazole vaginal suppositories did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects.
Other reported clinical experience has not identified differences in responses between the elderly and younger patients.
-
ADVERSE REACTIONS
Adverse Reactions from Clinical Trials
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
During controlled clinical studies conducted in the United States, 284 patients with vulvovaginal candidiasis were treated with terconazole 80 mg vaginal suppositories. Based on comparative analyses with placebo (295 patients), the adverse experiences considered adverse reactions most likely related to terconazole 80 mg vaginal suppositories were headache (30.3% vs. 20.7% with placebo) and pain of the female genitalia (4.2% vs. 0.7% with placebo). Adverse reactions that have also been reported but were not statistically significantly different from placebo were burning (15.2% vs. 11.2% with placebo) and body pain (3.9% vs. 1.7% with placebo). Fever (2.8% vs. 1.4% with placebo) and chills (1.8% vs. 0.7% with placebo) have also been reported. The adverse drug experience on terconazole most frequently causing discontinuation was burning (2.5% vs. 1.4% with placebo) and pruritus (1.8% vs. 1.4% with placebo).
Post-marketing Experience
The following adverse drug reactions have been first identified during post-marketing experience with Terconazole Vaginal Suppositories, 80 mg. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
General: Asthenia, Influenza-Like Illness consisting of multiple listed reactions including fever and chills, nausea, vomiting, myalgia, arthralgia, malaise
Immune: Hypersensitivity, Anaphylaxis, Face Edema
Nervous: Dizziness
Respiratory: Bronchospasm
Skin: Rash, Toxic Epidermal Necrolysis, Urticaria
-
OVERDOSAGE
In the rat, the oral LD 50 values were found to be 1741 and 849 mg/kg for the male and female, respectively. The oral LD 50 values for the male and female dog were ≅1280 and ≥640 mg/kg, respectively.
In the event of oral ingestion of suppository, supportive and symptomatic measures should be carried out.
-
DOSAGE AND ADMINISTRATION
One Terconazole Vaginal Suppository, 80 mg should be administered intravaginally once daily at bedtime for three consecutive days.
Before prescribing another course of therapy, the diagnosis should be reconfirmed by smears and/or cultures and other pathogens commonly associated with vulvovaginitis ruled out. The therapeutic effect of terconazole vaginal suppositories is not affected by menstruation.
- HOW SUPPLIED
- STORAGE
- SPL UNCLASSIFIED SECTION
-
Consumer Information Leaflet
TERCONAZOLE VAGINAL SUPPOSITORIES, 80 mg
Three oval suppositories, for use inside the vagina only.
Designed to be inserted into the vagina.
HOW TO USE:
Place one suppository into the vagina each night at bedtime, for 3 nights, as directed by your doctor.
The terconazole vaginal suppository is self-lubricating and may be inserted with or without the applicator.
A. Insertion with the applicator
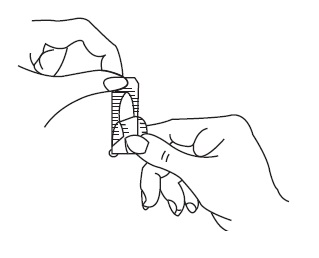
1. Filling the applicator
- •
- Break off suppository from the foil strip.
- •
- Pull the foil completely apart.
- •
- Place the flat end of the suppository into the open end of the applicator as shown.
- You are now ready to insert the suppository into the vagina.
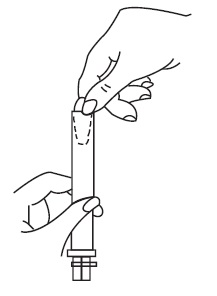
2. Using the applicator
- •
- Lie on your back with your knees drawn up toward your chest.
- •
- Holding the applicator by the ribbed end of the barrel, gently insert it into the vagina as far as it will comfortably go.
- •
- Press the plunger to release the suppository into the vagina.
- •
- Remove the applicator from the vagina.
3. Cleaning the applicator
After each use, you should thoroughly clean the applicator by following the procedure below:
- •
- Pull the plunger out of the barrel.
- •
- Wash both pieces with lukewarm, soapy water, and dry them thoroughly.
- •
- Put the applicator back together by gently pushing the plunger into the barrel as far as it will go.
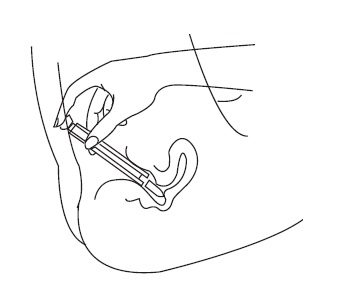
B. Insertion without the applicator
- •
- Lie on your back with your knees drawn up toward your chest.
- •
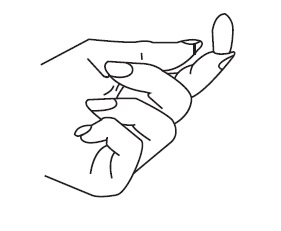
- Place the suppository on the tip of your finger as shown.
- •
- Insert the suppository gently into the vagina as far as it will comfortably go.
STORAGE:
Store at 20-25°C (68-77°F) [see USP Controlled Room Temperature].
See end flap for lot number and expiration date.
A WORD ABOUT YEAST INFECTIONS
Why do yeast infections occur?
Yeast infections are caused by an organism called Candida (KAN di duh). It may be present in small and harmless amounts in the mouth, digestive tract, and vagina. Sometimes the natural balance of the vagina becomes upset. This may lead to rapid growth of Candida, which results in a yeast infection. Symptoms of a yeast infection include itching, burning, redness, and an abnormal discharge.
Your doctor can make the diagnosis of a yeast infection by evaluating your symptoms and looking at a sample of the discharge under the microscope.
How can I prevent yeast infections?
Certain factors may increase your chance of developing a yeast infection. These factors don’t actually cause the problem, but they may create a situation that allows the yeast to grow rapidly.
- •
- Clothing: Tight jeans, nylon underwear, pantyhose, and wet bathing suits can hold in heat and moisture (two conditions in which yeast organisms thrive). Looser pants or skirts, 100% cotton underwear, and stockings may help avoid this problem.
- •
- Diet: Cutting down on sweets, milk products, and artificial sweeteners may reduce the risk of yeast infections.
- •
- Antibiotics: Antibiotics work by eliminating disease-causing organisms. While they are helpful in curing other problems, antibiotics may lead to an overgrowth of Candida in the vagina.
- •
- Pregnancy: Hormonal changes in the body during pregnancy encourage the growth of yeast. This is a very common time for an infection to occur. Until the baby is born, it may be hard to completely eliminate yeast infections. If you believe you are pregnant, tell your doctor.
- •
- Menstruation: Sometimes monthly changes in hormone levels may lead to yeast infections.
- •
- Diabetes: In addition to heat and moisture, yeast thrives on sugar. Because diabetics often have sugar in their urine, their vaginas are rich in this substance. Careful control of diabetes may help prevent yeast infection.
Controlling these factors can help eliminate yeast infections and may prevent them from coming back.
Some other helpful tips:
- 1.
- For best results, be sure to use the medication as prescribed by your doctor, even if you feel better quickly.
- 2.
- Avoid sexual intercourse, if your doctor advises you to do so. The suppository formulation may damage the diaphragm or latex condom. Therefore, use of the diaphragm or latex condom during therapy with the suppository is not recommended. Consult your physician.
- 3.
- If your partner has any penile itching, redness, or discomfort, he should consult his physician and mention that you are being treated for a yeast infection.
- 4.
- You can use the medication even if you are having your menstrual period. However, you should not use tampons because they may absorb the medication. Instead, use external pads or napkins until you have finished your medication. You may also wish to wear a sanitary napkin if the vaginal medication leaks.
- 5.
- Dry the genital area thoroughly after showering, bathing, or swimming. Change out of a wet bathing suit or damp exercise clothes as soon as possible. A dry environment is less likely to encourage the growth of yeast.
- 6.
- Wipe from front to rear (away from the vagina) after a bowel movement.
- 7.
- Don’t douche unless your doctor specifically tells you to do so. Douching may disturb the vaginal balance.
- 8.
- Don’t scratch if you can help it. Scratching can cause more irritation and spread the infection.
- 9.
- Discuss with your physician any medication you are already taking. Certain types of medication can make your vagina more susceptible to infection.
- 10.
- Eat nutritious meals to promote your general health.
Manufactured By Perrigo plc
Bronx, NY 10457
Distributed By
PadagisTM
Allegan, MI 49010
www.padagis.com
Rev 05-22
2X300 RC J10
- Package/Label Display Panel
-
INGREDIENTS AND APPEARANCE
TERCONAZOLE
terconazole suppositoryProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:45802-717 Route of Administration VAGINAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TERCONAZOLE (UNII: 0KJ2VE664U) (TERCONAZOLE - UNII:0KJ2VE664U) TERCONAZOLE 80 mg Inactive Ingredients Ingredient Name Strength MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) BUTYLATED HYDROXYANISOLE (UNII: REK4960K2U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:45802-717-08 3 in 1 CARTON 08/28/2006 1 NDC:45802-717-00 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA077149 08/28/2006 Labeler - Padagis Israel Pharmaceuticals Ltd (600093611)