Label: EYEWASH- water solution
- NDC Code(s): 13985-609-04
- Packager: MWI
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated October 26, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
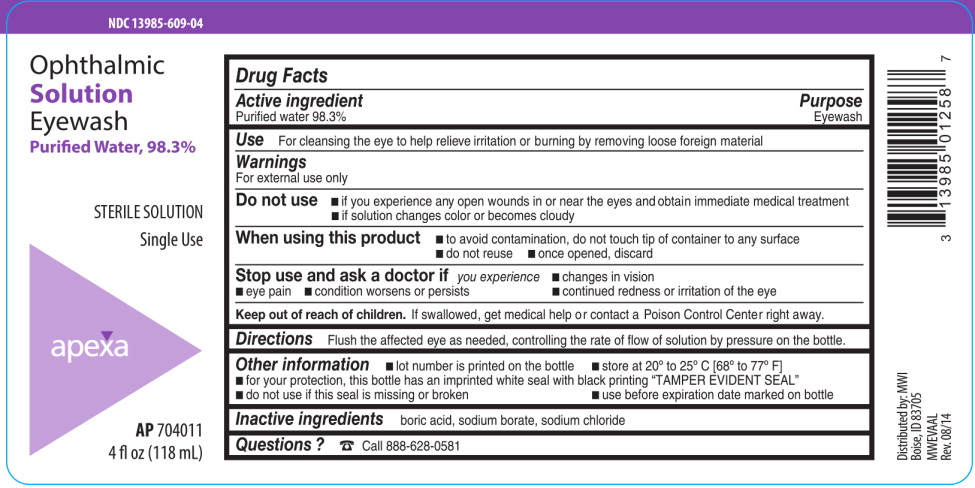
- Active ingredient
- Purpose
- Use
-
Warnings
For external use only
Do not use
- if you experience any open wounds in or near the eyes and obtain immediate medical treatment
- if solution changes color or becomes cloudy
When using this product
- to avoid contamination, do not touch tip of container to any surface
- do not reuse
- once opened, discard
- Directions
- Other information
- Inactive ingredients
- Questions ?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
EYEWASH
water solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13985-609 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WATER (UNII: 059QF0KO0R) (WATER - UNII:059QF0KO0R) WATER 929 g in 946 mL Inactive Ingredients Ingredient Name Strength BORIC ACID (UNII: R57ZHV85D4) SODIUM BORATE (UNII: 91MBZ8H3QO) SODIUM CHLORIDE (UNII: 451W47IQ8X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13985-609-04 1 in 1 BOTTLE 03/25/2015 1 118 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022305 02/23/2015 Labeler - MWI (019926120) Registrant - Akorn Operating Company LLC (117693100) Establishment Name Address ID/FEI Business Operations Niagara Pharmaceuticals, Inc. 205477792 manufacture(13985-609)