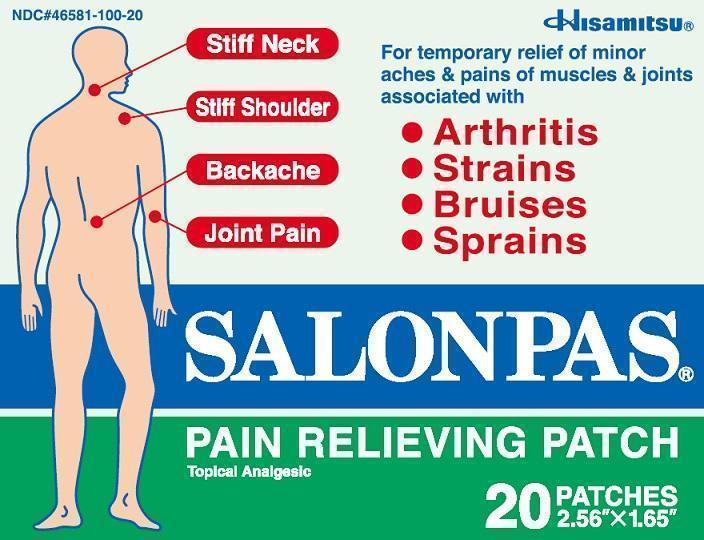
SALONPAS PAIN RELIEVING- camphor, menthol, methyl salicylate patch
Hisamitsu Pharmaceutical Co., Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Uses
For temporary relief of minor aches and pains of muscles and joints associated with:
- arthritis
- simple backache
- strains
- bruises
- sprains
Warnings
For external use only
Allergy alert:
If prone to allergic reaction from aspirin or salicylates, consult a doctor before use.
When using this product
- do not use otherwise than as directed
- avoid contact with the eyes, mucous membranes or rashes
- do not bandage tightly
Directions
Adults and children 12 years of age and over:
- clean and dry affected area
- remove patch from film
- apply to affected area not more than 3 to 4 times daily
- remove patch from the skin after at most 8 hours' application
Children under 12 years of age: consult a doctor
| SALONPAS PAIN RELIEVING
camphor, menthol, methyl salicylate patch |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Hisamitsu Pharmaceutical Co., Inc. (690539713) |
Revised: 12/2018
Document Id: 804b617d-8a17-4e60-9278-3f52bb1a1521
Set id: 1ebe6a18-5247-4172-93ee-161416db060d
Version: 7
Effective Time: 20181213
Hisamitsu Pharmaceutical Co., Inc.