LIPODOX- doxorubicin hydrochloride injectable, liposomal
LIPODOX 50- doxorubicin hydrochloride injectable, liposomal
Sun Pharmaceutical Industries, Inc.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
For the use of an Oncologist or a Cancer Hospital or a Laboratory only.
Doxorubicin Hydrochloride Liposome Injection
LIPODOX LIPODOX 50
(Pegylated Liposomal)
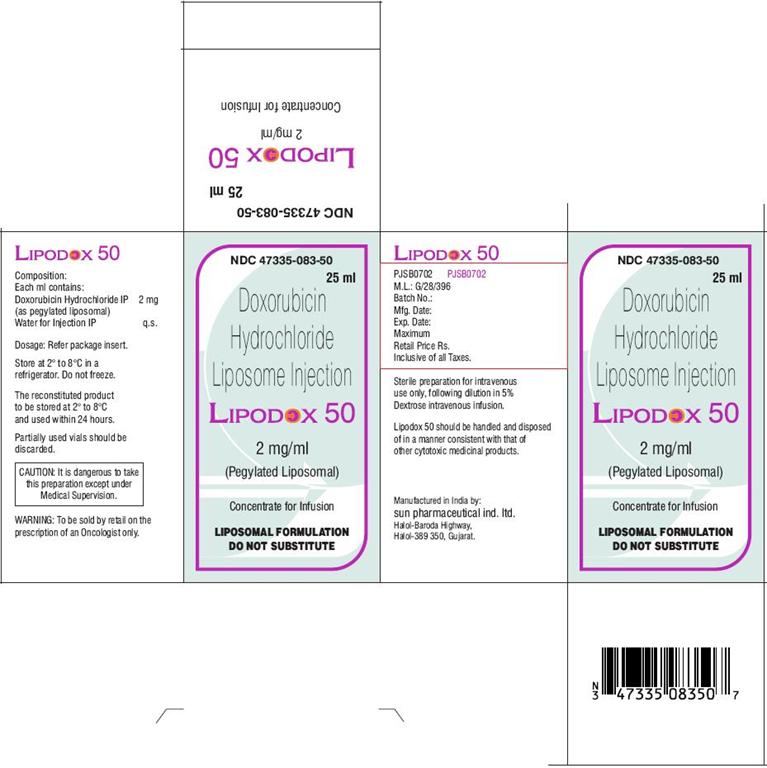
Composition:
Each ml contains:
Doxorubicin Hydrochloride IP 2 mg
(as pegylated liposomal)
Water for Injection IP q.s.
Lipodox is provided as a sterile, transluscent, red dispersion in single use vials.
Description:
Lipodox is doxorubicin hydrochloride encapsulated in long circulating pegylated Liposomes. Liposomes are microscopic vesicles composed of a phospholipid bilayer that are capable of encapsulating active drugs. The pegylated Liposomes of doxorubicin are formulated with surface bound methoxypolyethylene glycol (MPEG), a process often referred to as pegylation, to protect liposomes from detection by the mononuclear phagocyte system (MPS) and to increase blood circulation time.
Pegylated liposomes have a half life of approximately 55 hours in humans. They are stable in blood and direct measurement of liposomal doxorubicin shows that atleast 90% of the drug remains liposome encapsulated during circulation.
It is hypothesized that because of their small size and persistence in the circulation, the pegylated doxorubicin liposomes are able to penetrate the altered and often compromised vasculature of tumors. Once the pegylated liposomes distribute to the tissue compartment, the encapsulated doxorubicin HCL becomes available. The exact mechanism of release is not understood.
Clinical Pharmacology
Doxorubicin is a cytotoxic anthracycline antibiotic isolated from Streptomyces peucetius var. caesius. It is indicated for the treatment of metastatic carcinoma of the ovary, metastatic breast cancer and AIDS related Kaposis Sarcoma (KS).
Mechanism of Action
The exact mechanism of the antitumor activity of doxorubicin is not known. It is generally believed that inhibition of synthesis of DNA, RNA and protein is responsible for the majority of the cytotoxic effects. Liposomal doxorubicin penetrates the cells rapidly, binds to chromatin and inhibits nucleic acid synthesis by intercalation between adjacent base pairs of the DNA double helix thus preventing their unwinding for replication.
Pharmacokinetics
Liposomal doxorubicin displayed linear pharmacokinetics over the dose range of 10 to 20 mg/m2. Disposition occurred in two phases after doxorubicin administration with a relatively short phase (approximately 5 hours) and a prolonged second phase (approximately 55 hours) that accounted for the majority of the area under the curve (AUC).
The pharmacokinetics of liposomal doxorubicin at a dose of 50 mg/m2 is reported to be nonlinear. At this dose, the elimination half life of liposomal doxorubicin is expected to be longer and the clearance lower compared to a 20 mg/m2 dose. The exposure (AUC) is thus expected to be more than proportional at a 50 mg/m2 dose when compared with the lower doses.
The plasma protein binding of liposomal doxorubicin has not been determined; the plasma protein binding of doxorubicin is approximately 70%. Unlike conventional doxorubicin which displays a large volume of distribution, ranging from 700 to 1100 L/m2, a small steady state volume of distribution of liposomal doxorubicin shows that liposomal doxorubicin is confined mostly to the vascular fluid volume and the clearance of doxorubicin from the blood is dependent upon the liposomal carrier. Doxorubicin becomes available after the liposomes are extravasated and enter the tissue compartment.
The plasma clearance of liposomal doxorubicin was slow with a mean clearance of 0.041 L/h/m2 at a dose of 20 mg/m2. Because of the slow clearance, the AUC of liposome-encapsulated doxorubicin is approximately two to three orders of magnitude larger than the AUC for a similar dose of the conventional form of doxorubicin. Doxorubicinol, the main metabolite was detected at very low levels (0.8 to 26.2 ng/ml) in the plasma of patients who received liposomal doxorubicin at the dose of 10 to 20 mg/m2.
No pharmacokinetic study has been done in individuals with renal or hepatic insufficiency.
Indications:
Lipodox is indicated for the treatment of metastatic carcinoma of the ovary in patients with disease that is refractory to both paclitaxel and platinum based chemotherapy regimens. Refractory disease is defined as disease that has progressed while on treatment or within 6 months of completing treatment.
Lipodox is indicated as monotherapy for the treatment of metastatic breast cancer, where there is an increased cardiac risk.
Lipodox is also indicated for the treatment of AIDS related Kaposi’s Sarcoma in patients with extensive mucocutaneous or visceral disease that has progressed on prior combination therapy (consisting of two of the following agents: a vinca alkaloid, bleomycin and standard doxorubicin or another anthracycline) or in patients who are intolerant to such therapy.
Contraindications:
- •
- History of hypersensitivity reactions to the conventional formulation of doxorubicin or to any other components of this formulation.
- •
- Nursing mothers.
Warnings and Precautions:
Experience with large cumulative doses of liposomal doxorubicin is very limited. Liposomal doxorubicin’s cardiac risk and its risk compared to conventional doxorubicin formulations have not been adequately evaluated. At present, therefore, the warnings related to the use of conventional formulations of doxorubicin should be observed.
It is recommended that all patients receiving liposomal doxorubicin routinely undergo frequent ECG monitoring. Transient ECG changes such as, T-wave flattening, S-T segment depression and benign arrhythmias are not considered mandatory indications for the suspension of liposomal doxorubicin therapy. However, reduction of the QRS complex is considered more indicative of cardiac toxicity. If this change occurs, the most definitive test for anthracycline myocardial injury i.e., endomyocardial biopsy, must be considered.
More specific methods for the evaluation and monitoring of cardiac functions as compared to ECG are a measurement of left ventricular ejection fraction by echocardiography or preferably by multigated angiography (MUGA). These methods must be applied routinely before the initiation of liposomal doxorubicin therapy and repeated periodically during treatment. The evaluation of left ventricular function is considered to be mandatory before each additional administration of liposomal doxorubicin that exceeds a lifetime cumulative anthracycline dose of 450 mg/m2.
Whenever cardiomyopathy is suspected i.e., the left ventricular ejection fraction has substantially decreased relative to pretreatment values and/or left ventricular ejection fraction is lower than a prognostically relevant value (e.g., < 45%), endomyocardial biopsy may be considered and the benefit of continued therapy must be carefully evaluated against the risk of developing irreversible cardiac damage.
The evaluation tests and methods mentioned above concerning the monitoring of cardiac performance during anthracycline therapy are to be employed in the following order: ECG monitoring, measurement of left ventricular ejection fraction, endomyocardial biopsy. If a test result indicates possible cardiac injury associated with liposomal doxorubicin therapy, the benefit of continued therapy must be carefully weighed against the risk of myocardial injury.
Caution should be observed in patients who have received other anthracyclines and the total dosage of doxorubicin hydrochloride given should take into account any previous or concomitant therapy with other anthracyclines or related compounds. Cardiac toxicity may also occur at cumulative anthracycline doses lower than 450 mg/m2 in patients with prior mediastinal irradiation or in those receiving concurrent cyclophosphamide therapy.
Congestive cardiac failure due to cardiomyopathy may occur suddenly, without prior ECG changes and may also be encountered several weeks after discontinuation of therapy. Patients with a history of cardiovascular disease should be administered liposomal doxorubicin only when the potential benefit of treatment outweighs the risk.
Acute infusion related reactions characterized by flushing, shortness of breath, facial swelling, headache, chills, chest pain, back pain, tightness in the chest and throat, fever, tachycardia, pruritus, rash, cyanosis, syncope, bronchospasm, asthma, apnea and/or hypotension have been reported with liposomal doxorubicin. In most patients these reactions resolve over the course of several hours to a day once the infusion is terminated or when the rate of infusion is slowed.
Liposomal doxorubicin should be administered at the initial rate of 1 mg/min to minimize the risk of infusion reactions.
Serious and sometimes life threatening or fatal allergic/anaphylactoid like infusion reactions have been reported. Medications to treat such reactions and emergency equipment should be available for immediate use.
Moderate and reversible myelosupression has been observed in ovarian and breast cancer patients who received liposomal doxorubicin with anemia being the most common hematologic adverse event followed by leucopenia, thrombocytopenia and neutropenia.
Myelosuppression can be a dose limiting adverse event in patients with AIDS associated with Kaposi s sarcoma who already present with baseline myelosuppression. Again leucopenia seemed to be the most common haematological adverse event in this population.
Because of the potential for bone marrow suppression, careful hematologic monitoring including white blood cell, neutrophil, platelet counts and hemoglobin/hematocrit should be done. Hematologic toxicity may require dose reduction or delay or suspension of therapy. Persistent severe myelosuppression may result in superinfection, neutropenic fever or hemorrhage. Development of sepsis in the setting of neutropenia has resulted in the discontinuation of treatment and in rare cases death. Hematologic toxicity may be more severe when liposomal doxorubicin is administered in combination with other agents that cause bone marrow suppression. Dosage should be reduced in patients with impaired hepatic function.
Prior to liposomal doxorubicin administration evaluation of hepatic function is recommended using conventional clinical laboratory tests such as SGOT, SGPT, alkaline phosphatase and bilirubin.
Radiation induced toxicity to the myocardium, mucosae, skin and liver have been reported to be increased by the administration of doxorubicin HCl.
Given the difference in pharmacokinetic profiles and dosing schedules, liposomal doxorubicin should not be used interchangeably with other formulations of doxorubicin hydrochloride.
Pregnancy & Lactation
Liposomal doxorubicin is embryotoxic at doses of 1 mg/kg/day in rats and embryotoxic and abortifacient at 0.5 mg/kg/day in rabbits (both doses are about one eighth the 50 mg/m2 human dose on a mg/m2 basis).
There are no adequate and well controlled studies in pregnant women. If Lipodox is to be used during pregnancy, or if the patient becomes pregnant during therapy, the patient should be apprised of the potential hazard to the fetus. If pregnancy occurs during the first few months following treatment with Lipodox, the prolonged half life of the drug must be considered. Women of child bearing potential should be advised to avoid pregnancy.
It is not known whether this drug is excreted in human milk. Because many drugs, including anthracyclines, are excreted in breast milk and because of the potential of serious adverse effects in nursing infants from Lipodox, mothers should discontinue nursing prior to taking this drug.
Drug Interactions:
Although no formal studies have been done with liposomal doxorubicin, caution should be exercised in the concomitant use of drugs known to interact with the conventional form of doxorubicin.
Liposomal doxorubicin, like other doxorubicin hydrochloride preparations, may potentiate the toxicity of other anticancer therapies. During clinical trials in patients with solid tumors (including breast and ovarian cancer) who have received concomitant cyclophosphamide or taxanes, no new additive toxicities were noted.
Exacerbation of cyclophosphamide induced hemorrhagic cystitis and enhancement of hepatotoxicity of 6-mercaptopurine have also been reported with standard doxorubicin hydrochloride.
Caution is also advised when giving any other cytotoxic agents especially myelotoxic agents at the same time.
Side effects:
Ovarian cancer patients/Breast cancer patients: Adverse effects reported in 5% of patients include hematological adverse events such as leucopenia, neutropenia, anemia, thrombocytopenia and the non hematological adverse events such as palmar-plantar erythrodysesthesia (all grades), stomatitis (all grades), nausea (all grades), asthenia, vomiting, rash, alopecia, constipation, anorexia, mucous membrane disorder, diarrhea, abdominal pain, paresthesia, pain, fever, pharyngitis, dry skin, headache, dyspepsia, somnolence and skin discolouration.
The adverse effects reported in 1-5% of ovarian cancer patients are allergic reaction, chills, infection, chest pain, back pain, enlarged abdomen, malaise, oral moniliasis, mouth ulceration, esophagitis, dysphagia, peripheral edema, dehydration, myalgia, dizziness, depression, insomnia, anxiety, dyspnea, increased cough, rhinitis, pruritus, skin disorder, exfoliative dermatitis, herpes zoster, sweating, conjunctivitis and taste perversion.
The adverse effects reported in 1-5% of breast cancer patients are breast pain, leg cramps, edema, leg edema, peripheral neuropathy, oral pain, ventricular arrhythmia, folliculitis, bone pain, musculoskeletal pain, cold sores (non herpetic), fungal infection, epistaxis, upper respiratory tract infection, bullous eruption, dermatitis, erythematous rash, nail disorder, scaly skin, lacrimation and blurred vision.
AIDS-KS patients: Adverse effects associated with the discontinuation of treatment are bone marrow suppression, cardiac adverse events, infusion related reactions, toxoplasmosis, palmar-plantar erythrodysesthesia, pneumonia, cough/dyspnea, fatigue, optic neuritis, progression of a non-KS tumour and allergy to penicillins.
Adverse reactions reported in ≥ 5% of patients include hematological side effects such as neutropenia, anemia, thrombocytopenia and non hematological side events such as nausea, asthenia, fever, alopecia, increased alkaline phosphatase, vomiting, hypochromic anemia, diarrhea, stomatitis and oral moniliasis.
Side effects reported in 1-5% of patients which may be possibly drug related are headache, back pain, infection, allergic reaction, chills, chest pain, hypotension, tachycardia, herpes simplex, rash, itching, mouth ulceration, glossitis, constipation, aphthous stomatitis, anorexia, dysphagia, abdominal pain, hemolysis, increased prothrombin time, increased SGPT, weight loss, hypocalcemia, hyperbilirubinemia, hyperglycemia, dyspnea, albuminuria, pneumonia, retinitis, emotional lability, dizziness and somnolence.
Overdosage:
Acute overdosage with doxorubicin causes increases in mucositis, leukopenia and thrombocytopenia.
Treatment of acute overdosage consists of treatment of the severely myelosuppressed patient with hospitalisation, antibiotics, platelet and granulocyte transfusions and symptomatic treatment of mucositis.
Dosage and Administration:
Breast Cancer/Ovarian cancer: Lipodox should be administered intravenously at a dose of 50 mg/m2 at an initial rate of 1 mg/min to minimize the risk of infusion reactions. If no infusion related adverse events are observed, the rate of infusion can be increased to complete administration of the drug over one hour. The patient should be dosed once every 4 weeks, for as long as the patient responds satisfactorily or tolerates treatment.
In those patients who experience an infusion reaction, the method of infusion should be modified as follows: 5% of the total dose should be infused slowly over the first 15 minutes. If tolerated without reaction, the infusion rate may then be doubled for the next 15 minutes. If tolerated, the infusion may then be completed over the next hour for a total infusion time of 90 minutes.
The median time to response in clinical trials was reported to be 4 months therefore, a minimum of 4 courses is recommended. To manage adverse effects such as PPE, stomatitis or hematologic toxicity the doses may be delayed or reduced. Concomitant or pretreatment with antiemetics should be considered.
AIDS-KS patients: Lipodox should be administered intravenously at a dose of 20 mg/m2 over 30 minutes once every three weeks for as long as the patient responds satisfactorily and tolerates treatment.
General information: Do not administer as a bolus injection or an undiluted solution. Rapid infusion may increase the risk of infusion related reactions. No compatibility data are available for liposomal doxorubicin and therefore, it is not recommended that it be mixed with other drugs.
If any signs and symptoms of extravasation are observed, the infusion must be immediately terminated and restarted in another vein. The application of ice over the site of extravasation for approximately 30 minutes may be helpful in alleviating the local reaction.
Lipodox must not be given by the intramuscular or the subcutaneous route.
Dose modification guidelines: There should be careful monitoring of the patient for toxicity. Adverse events such as PPE, hematologic toxicities and stomatitis may be managed by dose delays and adjustments. Following the first appearance of a grade 2 or higher adverse event, the dosing should be adjusted or delayed as described in the following tables. Once the dose has been reduced, it should not be increased at a later time.
|
PALMAR- PLANTAR ERYTHRODYSESTHESIA
|
|
|
Toxicity Grade
|
Dose Adjustment
|
|
1-(Mild erythema, swelling or desquamation not interfering with daily activities). |
Redose unless patient has experienced previous grade 3 or 4 toxicity. If so, delay upto 2 weeks and decrease dose by 25%. Return to original dose interval. |
|
2- (Erythema, desquamation or swelling interfering with but not precluding normal physical activities, small blisters or ulceration less than 2 cm in diameter). |
Delay dosing upto 2 weeks or until resolved to grade 0-1. If after 2 weeks there is no resolution, Lipodox should be discontinued. |
|
3- (Blistering, ulceration or swelling interfering with walking or normal daily activities; cannot wear regular clothing). |
Delay dosing upto 2 weeks or until resolved to grade 0-1. Decrease dose by 25% and return to original dose interval. If after 2 weeks there is no resolution, Lipodox should be discontinued. |
|
4- (Diffuse or local process causing infectious complications, or a bed ridden state or hospitalization). |
Delay dosing upto 2 weeks or until resolved to grade 0-1. Decrease dose by 25% and return to original dose interval. If after 2 weeks there is no resolution, Lipodox should be discontinued |
|
STOMATITIS
|
|
|
Toxicity Grade |
Dose Adjustment |
|
1- (Painless ulcers, |
Redose unless patient has experienced grade 3 or 4 toxicity. If so, delay upto 2 weeks and decrease dose by 25%. Return to original dose interval. |
|
2- (Painful erythema, |
Delay dosing upto 2 weeks or until resolved to grade 0-1. If after 2 weeks there is no resolution, Lipodox should be discontinued. |
|
3- (Painful erythema, edema or ulcers and cannot eat). |
Delay dosing upto 2 weeks or until resolved to grade 0-1. Decrease dose by 25% and return to original dose interval. If after 2 weeks there is no resolution, Lipodox should be discontinued. |
|
4- (Requires parenteral or enteral support). |
Delay dosing upto 2 weeks or until resolved to grade 0-1. Decrease dose by 25% and return to original dose interval. If after 2 weeks there is no resolution, Lipodox should be discontinued. |
|
HEMATOLOGICAL TOXICITY
|
|||
|
Grade
|
ANC
|
Platelets
|
Modification
|
|
1 |
1500 - 1900 |
75,000 - 150,000 |
Resume treatment with no dose reduction. |
|
2 |
1000 - <1500 |
50,000 - <75,000 |
Wait until ANC ≥ 1500 and platelets ≥ 75,000; redose with no dose reduction. |
|
3 |
500 - 999 |
25,000 - <50,000 |
Wait until ANC ≥ 1500 and platelets ≥ 75,000; redose with no dose reduction. |
|
4 |
<500 |
<25,000 |
Wait until ANC ≥ 1500 and platelets ≥ 75,000; redose at 25% dose reduction or continue full dose with cytokine support. |
Pediatric patients: Safety and effectiveness in patients less than 18 years of age is not established.
Elderly: No overall differences were observed between these subjects and younger subjects, but greater sensitivity of some older individuals cannot be ruled out.
Hepatic impairment: Liposomal doxorubicin pharmacokinetics determined in a small number of patients with elevated total bilirubin levels do not differ from patients with normal total bilirubin; however, until further experience is gained, the liposomal doxorubicin dosage in patients with impaired hepatic function should be reduced based on the experience from the breast and ovarian clinical trial programmes as follows: At initiation of therapy, if bilirubin is between 1.2-3.0 mg/dl, the first dose is reduced by 25%. If the bilirubin is > 3.0 mg/dl, the first dose is reduced by 50%.
If the patient tolerates the first dose without an increase in serum bilirubin or liver enzymes, the dose for cycle 2 can be increased to the next dose level, i.e., if reduced by 25% for the first dose, increase to full dose for cycle 2; if reduced by 50% for the first dose, increase to 75% of full dose for cycle 2. The dosage can be increased to full dose for subsequent cycles if tolerated. Liposomal doxorubicin can be administered to patients with liver metastases with concurrent elevation of bilirubin and liver enzymes upto 4 x the upper limit of the normal range. Prior to liposomal doxorubicin administration the hepatic function should be evaluated using conventional clinical laboratory tests such as ALT/AST, alkaline phosphatase and bilirubin.
Renal impairment: As doxorubicin is metabolised by the liver and excreted in the bile, dose modification will not be required. Population pharmacokinetic data (in the range of creatinine clearance of 30-156 ml/min) demonstrate that liposomal doxorubicin clearance is not influenced by renal function. No pharmacokinetic data are available in patients with creatinine clearance of less than 30 ml/min.
Preparation for intravenous administration: The appropriate dose of liposomal doxorubicin upto a maximum of 90 mg must be diluted in 250 ml of 5% Dextrose Injection USP prior to administration. Doses exceeding 90 mg should be diluted in 500 ml of 5% dextrose injection USP prior to administration. Aseptic technique must be strictly observed since no preservative or bacteriostatic agents is present in Lipodox. Diluted liposomal doxorubicin should be refrigerated at 2°C to 8°C and administered within 24 hours. Lipodox should not be used with in line filters and should not be mixed with other drugs. It should not be used with any other diluent other than Dextrose injection 5%. Partially used vials should be discarded.
Lipodox is not a clear solution but a transluscent, red liposomal dispersion.
Parenteral drug products should be inspected visually for particulate matter and discolouration prior to administration, whenever solution and container permit. Do not use if a precipitate or foreign matter is present.
Doxorubicin is not a vesicant but should be considered an irritant and precautions should be taken to avoid extravasation. With intravenous administration of liposomal doxorubicin, extravasation may occur with or without an accompanying stinging or burning sensation even if blood returns well on aspiration of the infusion needle. If any signs or symptoms of extravasation have occurred, the infusion should be immediately terminated and restarted in another vein. The application of ice over the side of extravasation for approximately 30 minutes may be helpful in alleviating the local reaction.
Caution should be exercised in the handling and preparation of liposomal doxorubicin. The use of gloves is required. If Lipodox comes into contact with skin or mucosa, immediately wash thoroughly with soap or water. It should be handled and disposed off in a manner consistent with other anticancer drugs.
Incompatibilities:
Lipodox should not be mixed with other drugs. It should not be used with any other diluent other than Dextrose injection 5%.
Presentation:
Lipodox is available as 2 mg/ml concentrate solution for infusion in 5 ml and 10 ml vials.
Lipodox 50 is available as 2 mg/ml concentrate solution for infusion in 30 ml vial containing 25 ml concentrate solution for infusion.
NDC:
Lipodox (10 ml): NDC 47335-082-50
Lipodox 50 (25 ml): NDC 47335-083-50
For further details, please write to:
Sun Pharmaceutical Ind. Ltd.
Acme Plaza, Andheri-Kurla Road,
Andheri (E), Mumbai-400 059, INDIA.
PJPI0383
D140/032010/V6
Dear Healthcare Provider letter
URGENT – Doxil® (Doxorubicin Hydrochloride Liposome Injection) Shortage Update
January 27, 2012
Dear Healthcare Professional,
Due to the current critical shortage of Doxil® (doxorubicin HCL liposome injection) [Janssen Products, LP: 2 mg/mL-20 mg/10 mL and 50 mg/25 mL] in the United States (US) market, Sun Pharma Global FZE (Sun Pharma) through Caraco Pharmaceutical Laboratories Ltd is coordinating with the Food and Drug Administration (FDA) to provide an alternative treatment option during this critical shortage period.
On behalf of Sun Pharma, Caraco Pharmaceutical Laboratories Ltd has initiated temporary importation of Lipodox™ (Doxorubicin Hydrochloride Liposome Injection) into the US market. Sun Pharma’s Lipodox™ Doxorubicin Hydrochloride Liposome Injection 20 mg/10 mL (2 mg/mL) single use vials and Lipodox 50™ (Doxorubicin Hydrochloride Liposome Injection) 50 mg/25 mL (2 mg/mL) single use vials contain the same active ingredient, doxorubicin hydrochloride, in the same concentration as Doxil® (doxorubicin HCL liposome injection) [Janssen Products, LP: 2 mg/mL-20 mg/10 mL and 50 mg/25 mL] marketed in the United States.
Sun Pharma’s Lipodox™ (Doxorubicin Hydrochloride Liposome Injection) 20 mg/10 mL (2 mg/mL) single use vials and Lipodox 50™ (Doxorubicin Hydrochloride Liposome Injection) 50 mg/25 mL (2 mg/mL) single use vials are manufactured in India at an FDA inspected facility, Sun Pharmaceutical Industries Limited – Halol, Gujarat.
Effective immediately, Sun Pharma will offer the following dosage forms:
|
Doxorubicin Hydrochloride Liposome Injection |
|
20 mg/10 mL (2 mg/mL) - 10 mL single use vials (Lipodox™) |
|
50 mg/25 mL (2 mg/mL) - 25 mL single use vials (Lipodox 50™) |
In the US, the labeling for Doxil® (Doxorubicin Hydrochloride Liposome Injection) includes the following indications: i) treatment of patients with ovarian cancer whose disease has progressed or recurred after platinum-based chemotherapy; ii) treatment of AIDS-related Kaposi’s sarcoma in patients after failure of prior systemic chemotherapy or intolerance to such therapy; iii) treatment of multiple myeloma in combination with bortezomib in patients who have not previously received bortezomib and have received at least one prior therapy.
Refer to Sun Pharma’s package insert for full prescribing information. Please note the important dosage and administration warning presented in Sun Pharma’s product labeling.
Sun Pharma’s Lipodox™ and Lipodox 50™ (Doxorubicin Hydrochloride Liposome Injection) should be handled exactly as you have handled the FDA approved Doxil® (doxorubicin HCL liposome injection). Refrigerate unopened vials of doxorubicin hydrochloride liposome injection at 2° to 8°C (36° to 46°F). Avoid freezing. Prolonged freezing may adversely affect liposomal drug products; however, short-term freezing (less than 1 month) does not appear to have a deleterious effect on doxorubicin hydrochloride liposome injection.
To order Sun Pharma’s Lipodox™ (Doxorubicin Hydrochloride Liposome Injection) 20 mg/10 mL (2 mg/mL) single use vials and Lipodox 50™ (Doxorubicin Hydrochloride Liposome Injection) 50 mg/25 mL (2 mg/mL) single use vials, please contact the shortage response team by phone 1888 835 2237 or fax 1800 980 2237.
To report adverse events or medication errors among patients administered Sun Pharma’s Lipodox™ or Lipodox 50™ (Doxorubicin Hydrochloride Liposome Injection), please contact our partner Caraco Pharmaceutical Laboratories Limited at 1-800-818-4555.
Adverse events may also be reported to the FDA’s MedWatch Adverse Event Reporting Program either online, by regular mail or by fax:
- •
- Online: www.fda.gov/medwatch/report.htm
- •
- Regular Mail: use postage-paid FDA form 3500 available at www.fda.gov/MedWatch/getforms.htm. Mail to: MedWatch, FDA, 5600 Fishers Lane, Rockville, MD 20852-9787
- •
- Fax: +1-800-FDA-0178
At this time, FDA’s regulatory discretion for the importation and distribution of Sun Pharma’s Lipodox™ (Doxorubicin Hydrochloride Liposome Injection) is limited to Sun Pharma Global FZE and its authorized distributor, Caraco Pharmaceutical Laboratories Ltd, during the critical shortage of Doxil. Importation or distribution of this product in the United States by any other entity is outside the scope of FDA’s regulatory discretion, and FDA has not approved Sun Pharma’s Lipodox™ product for marketing in the U.S.
Sincerely,
Vishwanath Kenkare
Manager
Sun Pharma Global FZE
| LIPODOX
doxorubicin hydrochloride injectable, liposomal |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LIPODOX 50
doxorubicin hydrochloride injectable, liposomal |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Sun Pharmaceutical Industries, Inc. (146974886) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Sun Pharmaceutical Industries Limited | 725959238 | MANUFACTURE(47335-082, 47335-083) , ANALYSIS(47335-082, 47335-083) | |