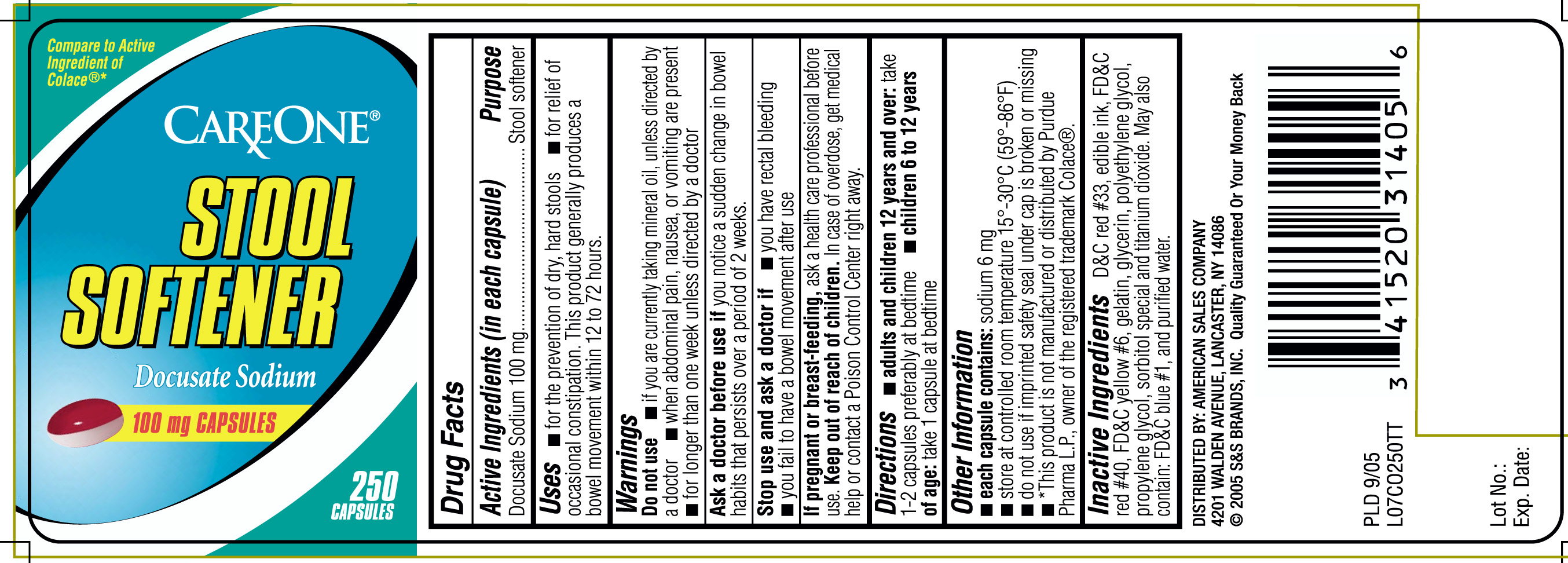
STOOL SOFTENER- docusate sodium capsule, liquid filled
Care One (American Sales Company)
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Drug Facts
Uses
- for the prevention of dry, hard stools
- for relief of occasional constipation. This product generally produces a bowel movement within 12 to 72 hours.
Warnings - Do not use
- if you are currently taking mineral oil, unless directed by a doctor
- when abdominal pain, nausea or vomiting are present
- for longer than one week, unless directed by a doctor
Directions
-
adults and children 12 years and over: take 1- 2 capsules preferably at bedtime
- children 6 to 12 years of age: take 1 capsule at bedtime
Other information
- each capsule contains: sodium 6 mg
- store at controlled room temperature 15o - 30o C (59o- 86o F)
- do not use if imprinted safety seal under cap is broken or missing.
- *This product is not manufactured or distributed by Purdue Pharma L.P., owner of the registered trademark Colace®
Inactive Ingredients
D&C Red #33, edible ink, FD&C Red #40, FD&C Yellow #6, gelatin, glycerin, polyethylene glycol, propylene glycol, sorbitol special and titanium dioxide. May also contain: FD&C blue #1 and purified water.
| STOOL SOFTENER
docusate sodium capsule, liquid filled |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Care One (American Sales Company) (809183973) |
| Registrant - P & L Development, LLC (800014821) |
Revised: 12/2012
Document Id: e12462d6-7d6c-4d7a-b057-11129e97f9a5
Set id: 1c5aa329-441c-40de-bda6-8132caf25ae9
Version: 2
Effective Time: 20121227
Care One (American Sales Company)