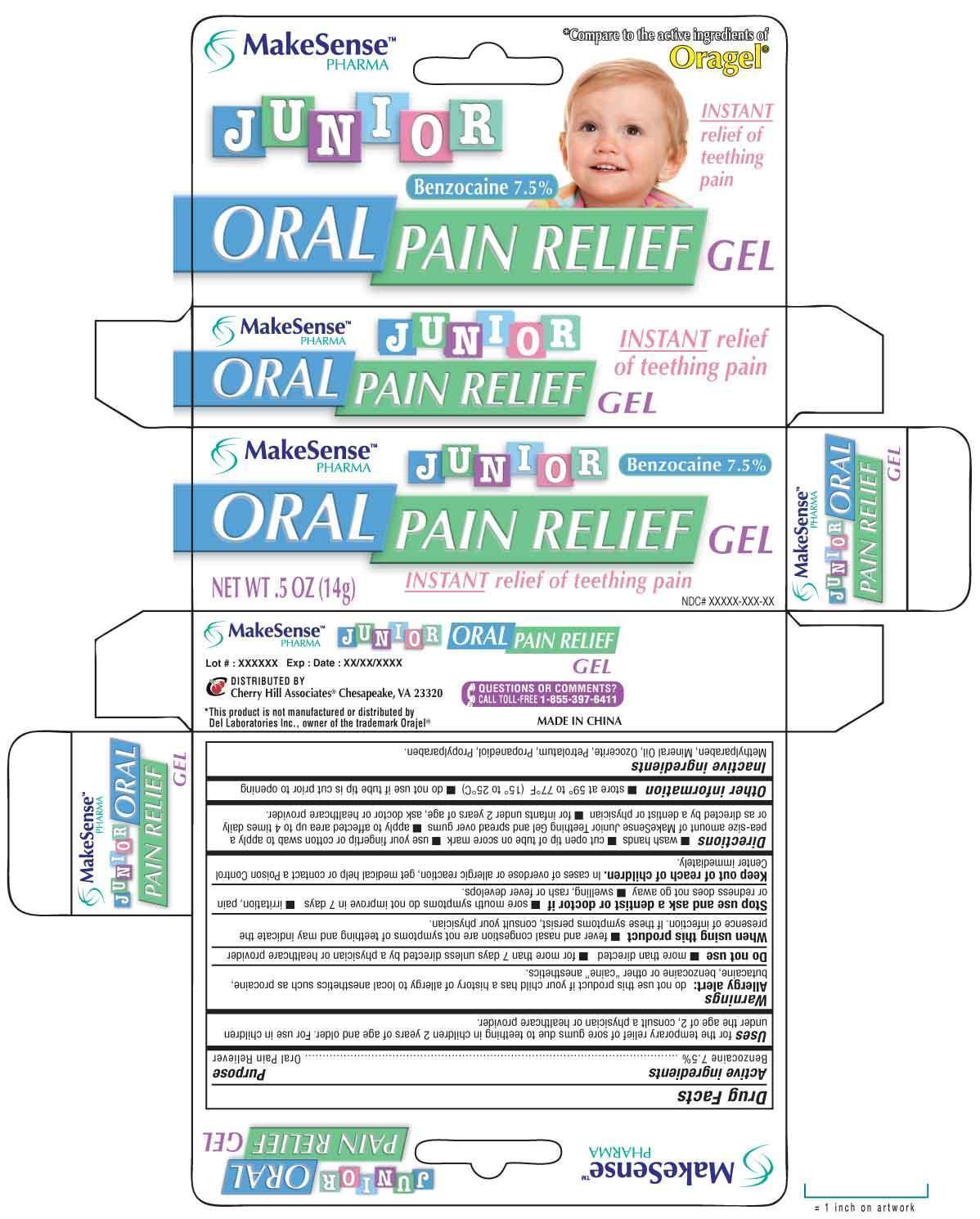
Label: MAKESENSE JUNIOR PAIN RELIEF- benzocaine gel
-
Contains inactivated NDC Code(s)
NDC Code(s): 69020-206-14 - Packager: Cherry Hill Sales Co
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated July 30, 2014
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Uses
- Warnings
- Do not use
- When using this product
- Stop use and ask a dentist or doctor if
- Keep out of reach of children
-
Directions
■ wash hands ■ cut open tip of tube on score mark ■ use your fingertip or cotton applicator to apply a small pea-size amount of MakeSense Junior Teething Gel and spread over gums ■ apply to affected area up to 4 times daily or as directed by a dentist or physician ■ for infants under 2 years of age, ask a doctor or health care provider
- Other information
- Inactive ingredients
- Package Label
-
INGREDIENTS AND APPEARANCE
MAKESENSE JUNIOR PAIN RELIEF
benzocaine gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69020-206 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOCAINE (UNII: U3RSY48JW5) (BENZOCAINE - UNII:U3RSY48JW5) BENZOCAINE 7.5 g in 100 g Inactive Ingredients Ingredient Name Strength METHYLPARABEN (UNII: A2I8C7HI9T) MINERAL OIL (UNII: T5L8T28FGP) CERESIN (UNII: Q1LS2UJO3A) PETROLATUM (UNII: 4T6H12BN9U) PROPANEDIOL (UNII: 5965N8W85T) PROPYLPARABEN (UNII: Z8IX2SC1OH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69020-206-14 14 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part356 07/15/2014 Labeler - Cherry Hill Sales Co (145959768)