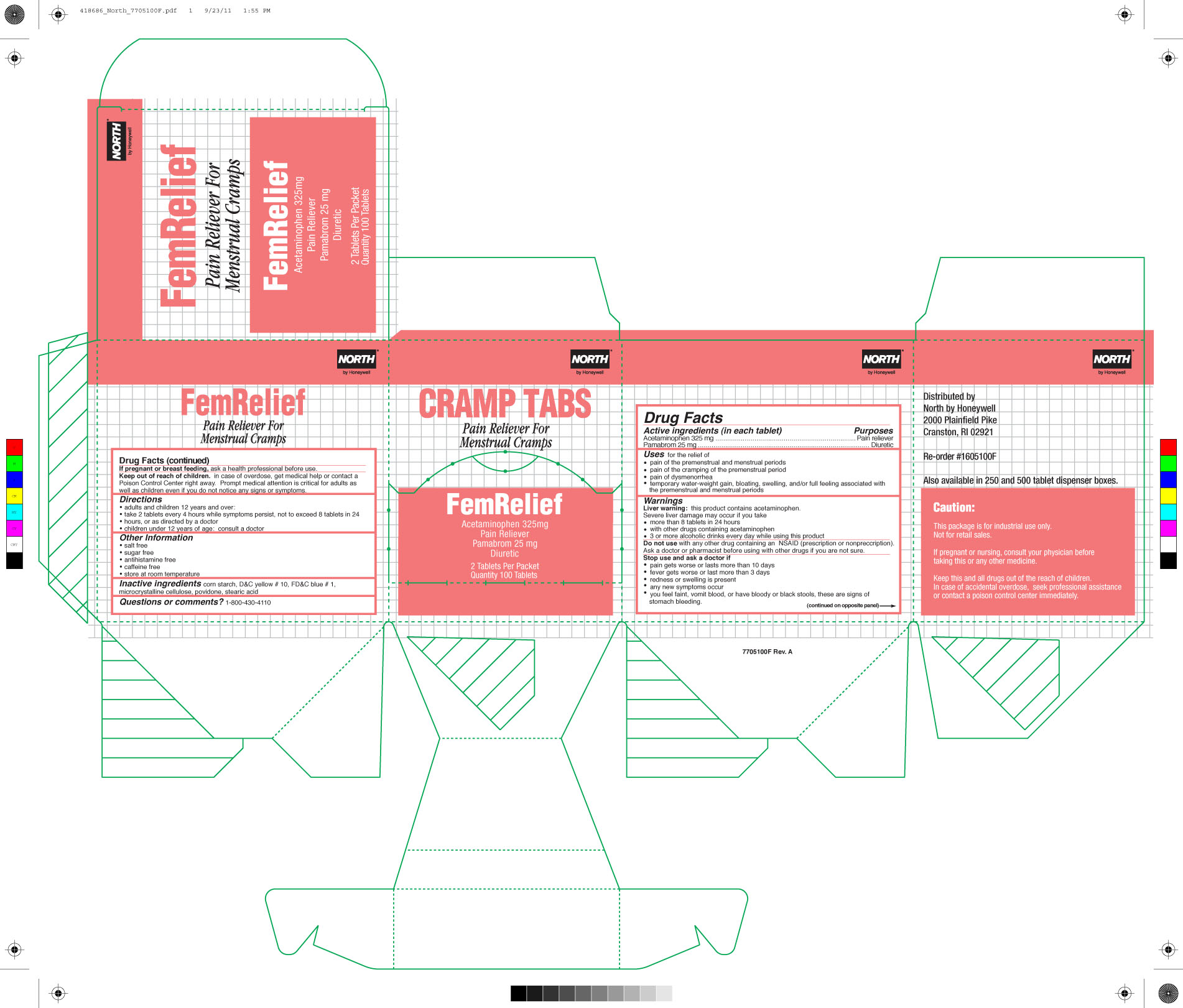
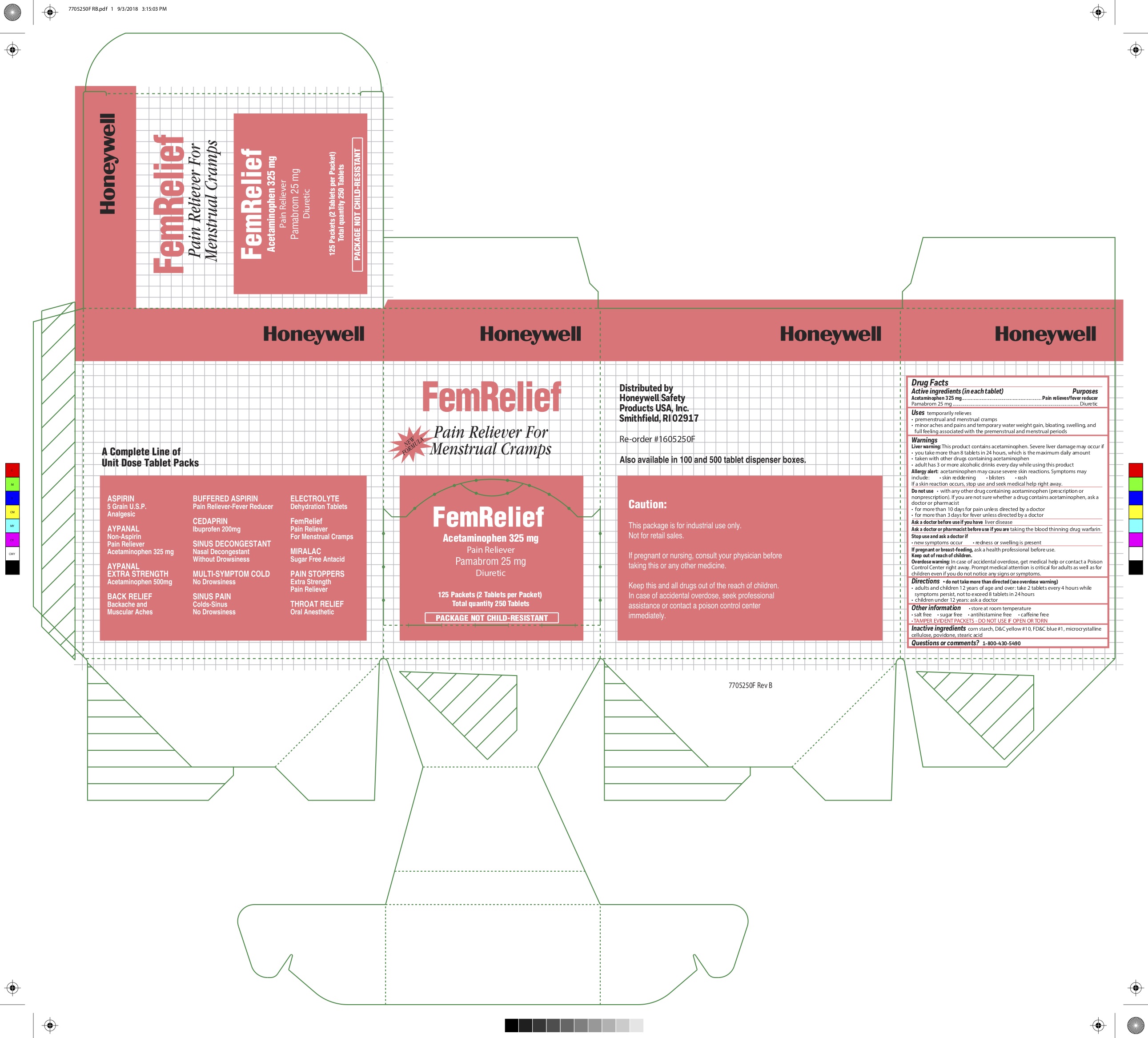
FEM RELIEF- acetaminophen pamabrom tablet
Honeywell Safety Products USA, Inc
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
0498-7001 & 0498-7000: Fem Relief
Uses
temporarily relieves
- premenstrual and menstrual cramps
- minor aches and pains and temporary weight gain, bloating, swelling, and full feeling associated with the premenstrual and menstrual periods
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take:
- more than 8 tablets in 24 hours, which is the maximum daily amount
- taken with other drugs containing acetaminophen
- adult has 3 or more alcoholic drinks every day while using this product
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Directions
- do not take more than directed (see overdose warning)
- Adults and Children 12 years of age and over: take 2 tablets with water every 4 hours while symptoms persist, not to exceed 8 tablets in 24 hours.
- Children under 12: consult a doctor
Other information
- store at room temperature
- salt free
- sugar free
- antihistamine free
- caffeine free
- TAMPER EVIDENT PACKETS-DO NOT USE IF OPEN OR TORN
| FEM RELIEF
acetaminophen pamabrom tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| FEM RELIEF
acetaminophen pamabrom tablet |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Honeywell Safety Products USA, Inc (118768815) |
Revised: 1/2024
Document Id: 0ecabbee-c5b4-f0ed-e063-6394a90a98a4
Set id: 1be326af-0a12-4a69-be5d-32bbffc6dbad
Version: 12
Effective Time: 20240112
Honeywell Safety Products USA, Inc