UREA- urea cream
Stratus Pharmaceuticals, Inc
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
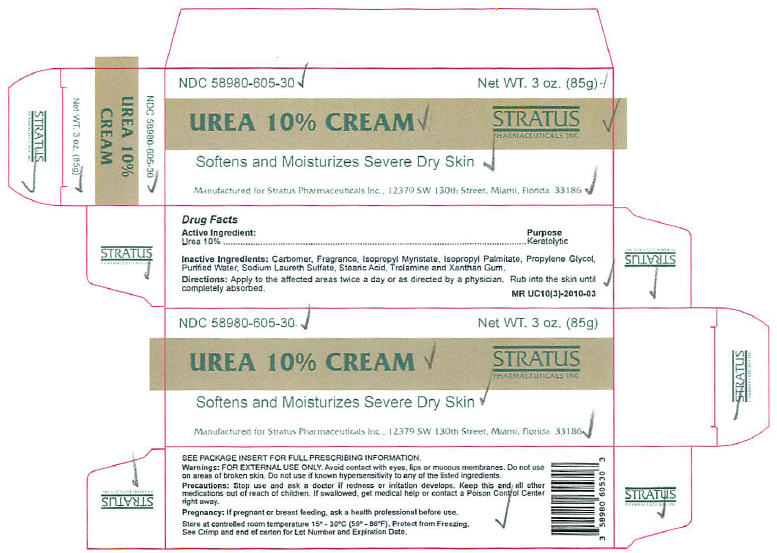
UREA 10% CREAM
DESCRIPTION
UREA 10% CREAM is a potent keratolytic emollient which is a gentle tissue softener for skin and/or nails.
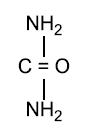
Each gram of UREA 10% CREAM contains 10% Urea in a cream base of Carbomer, Fragrance, Isopropyl Myristate, Isopropyl Palmitate, Propylene Glycol, Purified Water, Sodium Laureth Sulfate, Stearic Acid, Trolamine and Xanthan Gum.
CLINICAL PHARMACOLOGY
Urea gently dissolves the intercellular matrix which results in loosening the horny layer of skin and shedding scaly skin at regular intervals, thereby softening hyperkeratotic areas. Urea also hydrates and gently dissolves the intercellular matrix of the nail plate, which can result in the softening and eventual debridement of the nail plate.
INDICATIONS AND USES
For debridement and promotion of normal healing of hyperkeratotic surface lesions, particularly where healing is retarded by local infection, necrotic tissue, fibrinous or prurient debris or eschar. Urea is useful for the treatment of hyperkeratotic conditions such as dry, rough skin, dermatitis, psoriasis, xerosis, ichthyosis, eczema, keratosis, keratoderma, corns and calluses, as well as damaged, ingrown and devitalized nails.
PRECAUTIONS
If redness or irritation occurs, discontinue use. After applying this medication, wash hands and unaffected areas thoroughly. KEEP THIS AND ALL MEDICATION OUT OF THE REACH OF CHILDREN.
PREGNANCY
Pregnancy Category B
Animal reproduction studies have revealed no evidence of harm to the fetus, however, there are no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, UREA 10% CREAM should be given to a pregnant woman only if clearly needed.
ADVERSE REACTIONS
Transient stinging, burning, itching or irritation may occur and normally disappear on discontinuing the medication.
DOSAGE AND ADMINISTRATION
Apply UREA 10% CREAM to affected skin twice per day or as directed by a physician. Rub in until completely absorbed. Apply to diseased or damaged nail tissue twice per day or as directed by a physician.
| UREA
urea cream |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Stratus Pharmaceuticals, Inc (789001641) |