DULCOLAX- docusate sodium capsule, liquid filled
Boehringer Ingelheim Pharmaceuticals, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
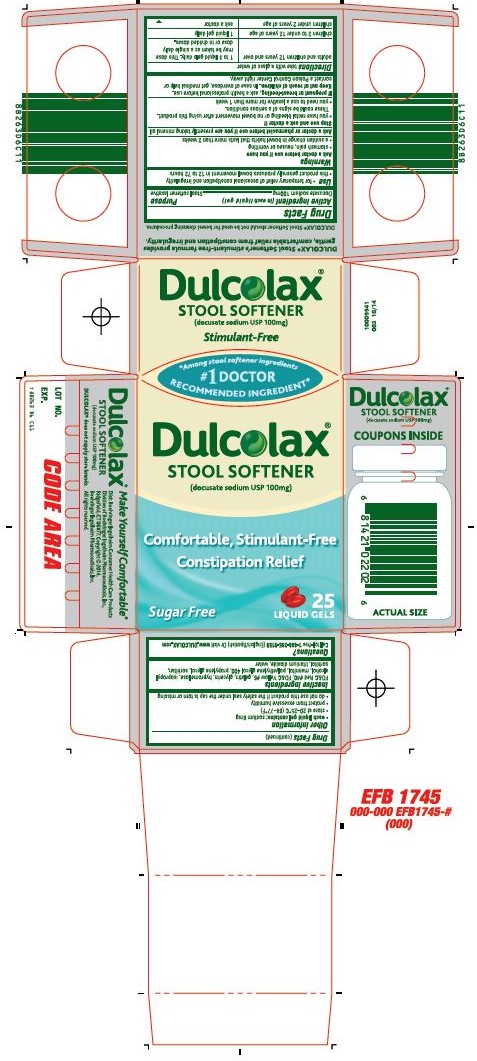
Dulcolax®
Stool Softener (docusate sodium
USP 100mg)
Ask a doctor before use if you have
- stomach pain, nausea or vomiting
- a sudden change in bowel habits that lasts more than 2 weeks
Directions take with a glass of water
| adults and children 12 years and over | 1 to 3 liquid gels daily. This dose may be taken as a single daily dose or in divided doses. |
| children 2 to under 12 years of age | 1 liquid gel daily |
| children under 2 years of age | ask a doctor |
- each liquid gel contains: sodium 6mg
- store at 20-25°C (68-77°F)
- protect from excessive humidity
- do not use this product if the safety seal under the cap is torn or missing
FD&C Red #40, FD&C Yellow #6, gelatin, glycerin, hypromellose, isopropyl alcohol, mannitol, polyethylene glycol 400, propylene glycol, sorbitan, sorbitol, titanium dioxide, water
Questions? Call toll-free 1-888-285-9159 (English/Spanish) Or visit www.DULCOLAX.com
Dist: Boehringer Ingelheim Consumer Health Care Products Division of Boehringer Ingelheim Pharmaceuticals, Inc., Ridgefield, CT 06877. Copyright © 2014, Boehringer Ingelheim Pharmaceuticals, Inc. All rights reserved.
| DULCOLAX
docusate sodium capsule, liquid filled |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Boehringer Ingelheim Pharmaceuticals, Inc. (603175944) |
| Registrant - Boehringer Ingelheim Pharmaceuticals, Inc. (603175944) |