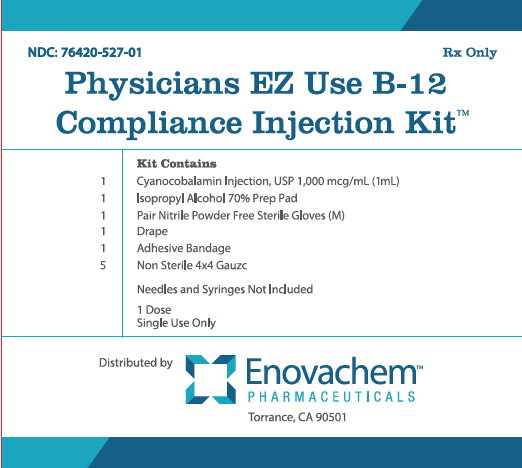
Label: PHYSICIANS EZ USE B-12 COMPLIANCE kit
- NDC Code(s): 63323-044-00, 76420-527-01
- Packager: Asclemed USA, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated September 22, 2020
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Isopropyl Alcohol 70% Prep Pads
- Active ingredient
- Purpose
- Uses
-
Warnings
For external use only
Flammable - keep away from fire or flame
When using this product do not
- get into eyes
- apply over large areas of the body
- in case of deep or puncture wounds, animal bites or serious burns consult a doctor
- Directions
- Other information
- Inactive ingredient
- Cyanocobalamin Injection, USP
-
DESCRIPTION
Cyanocobalamin Injection, USP is a sterile solution of cyanocobalamin for intramuscular or subcutaneous use.
Each mL contains 1000 mcg cyanocobalamin; sodium chloride 0.9%; benzyl alcohol 1.5%; Water for Injection q.s. Hydrochloric acid and/or sodium hydroxide for pH adjustment if necessary (4.5-7.0).
Cyanocobalamin appears as dark, red crystals or as an amorphous or crystalline, red powder. It is very hygroscopic in the anhydrous form, and sparingly soluble in water (1:80). It is stable to autoclaving for short periods at 121°C. The Vitamin B 12 coenzymes are very unstable in light.
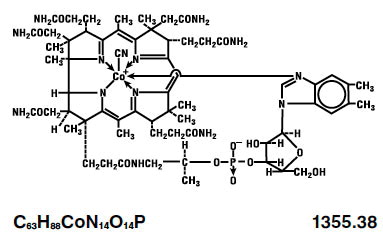
The chemical name is 5,6-dimethyl-benzimidazolyl cyanocobamide. The cobalt content is 4.34%. The structural formula is represented below:
-
CLINICAL PHARMACOLOGY
Vitamin B 12 is essential to growth, cell reproduction, hematopoiesis, nucleoprotein and myelin synthesis.
Cyanocobalamin is quantitatively and rapidly absorbed from intramuscular and subcutaneous sites of injection; the plasma level of the compound reaches its peak within one hour after intramuscular injection. Absorbed Vitamin B 12 is transported via specific B 12 binding proteins, transcobalamin I and II to the various tissues. The liver is the main organ for Vitamin B 12 storage.
Within 48 hours after injection of 100 or 1000 mcg of Vitamin B 12, 50 to 98% of the injected dose may appear in the urine. The major portion is excreted within the first eight hours. Intravenous administration results in even more rapid excretion with little opportunity for liver storage.
Gastrointestinal absorption of Vitamin B 12 depends on the presence of sufficient intrinsic factor and calcium ions. Intrinsic factor deficiency causes pernicious anemia, which may be associated with subacute combined degeneration of the spinal cord. Prompt parenteral administration of Vitamin B 12 prevents progression of neurologic damage.
The average diet supplies about 5 to 15 mcg/day of Vitamin B 12 in a protein-bound form that is available for absorption after normal digestion. Vitamin B 12 is not present in foods of plant origin, but is abundant in foods of animal origin. In people with normal absorption, deficiencies have been reported only in strict vegetarians who consume no products of animal origin (including no milk products or eggs).
Vitamin B 12 is bound to intrinsic factor during transit through the stomach; separation occurs in the terminal ileum in the presence of calcium, and Vitamin B 12 enters the mucosal cell for absorption. It is then transported by the transcobalamin binding proteins. A small amount (approximately 1% of the total amount ingested) is absorbed by simple diffusion, but this mechanism is adequate only with very large doses. Oral absorption is considered too undependable to rely on in patients with pernicious anemia or other conditions resulting in malabsorption of Vitamin B 12.
Cyanocobalamin is the most widely used form of Vitamin B 12, and has hematopoietic activity apparently identical to that of the antianemia factor in purified liver extract. Hydroxocobalamin is equally as effective as cyanocobalamin, and they share the cobalamin molecular structure.
-
INDICATIONS AND USAGE
Cyanocobalamin is indicated for Vitamin B 12 deficiencies due to malabsorption which may be associated with the following conditions:
Addisonian (pernicious) anemia
Gastrointestinal pathology, dysfunction, or surgery, including gluten enteropathy or sprue, small bowel bacterial overgrowth, total or partial gastrectomy
Fish tapeworm infestation
Malignancy of pancreas or bowel
Folic acid deficiency
It may be possible to treat the underlying disease by surgical correction of anatomic lesions leading to small bowel bacterial overgrowth, expulsion of fish tapeworm, discontinuation of drugs leading to vitamin malabsorption (see Drug/Laboratory Test Interactions), use of a gluten-free diet in nontropical sprue, or administration of antibiotics in tropical sprue. Such measures remove the need for long-term administration of cyanocobalamin.
Requirements of Vitamin B 12 in excess of normal (due to pregnancy, thyrotoxicosis, hemolytic anemia, hemorrhage, malignancy, hepatic and renal disease) can usually be met with oral supplementation.
Cyanocobalamin injection is also suitable for the Vitamin B 12 absorption test (Schilling test).
- CONTRAINDICATIONS
-
WARNINGS
WARNING: This product contains aluminum that may be toxic. Aluminum may reach toxic levels with prolonged parenteral administration if kidney function is impaired. Premature neonates are particularly at risk because their kidneys are immature, and they require large amounts of calcium and phosphate solutions, which contain aluminum.
Research indicates that patients with impaired kidney function, including premature neonates, who receive parenteral levels of aluminum at greater than 4 to 5 mcg/kg/day accumulate aluminum at levels associated with central nervous system and bone toxicity. Tissue loading may occur at even lower rates of administration.
Patients with early Leber’s disease (hereditary optic nerve atrophy) who were treated with cyanocobalamin suffered severe and swift optic atrophy.
Hypokalemia and sudden death may occur in severe megaloblastic anemia which is treated intensely.
Anaphylactic shock and death have been reported after parenteral Vitamin B 12 administration. An intradermal test dose is recommended before cyanocobalamin injection is administered to patients suspected of being sensitive to this drug.
This product contains benzyl alcohol. Benzyl alcohol has been reported to be associated with a fatal ‘‘Gasping Syndrome’’ in premature infants.
-
PRECAUTIONS
General
Vitamin B 12 deficiency that is allowed to progress for longer than three months may produce permanent degenerative lesions of the spinal cord. Doses of folic acid greater than 0.1 mg/day may result in hematologic remission in patients with Vitamin B 12 deficiency. Neurologic manifestations will not be prevented with folic acid, and if not treated with Vitamin B 12, irreversible damage will result.
Doses of cyanocobalamin exceeding 10 mcg daily may produce hematologic response in patients with folate deficiency. Indiscriminate administration may mask the true diagnosis.
Information for Patients
Patients with pernicious anemia should be instructed that they will require monthly injections of Vitamin B 12 for the remainder of their lives. Failure to do so will result in return of the anemia and in development of incapacitating and irreversible damage to the nerves of the spinal cord. Also, patients should be warned about the danger of taking folic acid in place of Vitamin B 12, because the former may prevent anemia but allow progression of subacute combined degeneration.
A vegetarian diet which contains no animal products (including milk products or eggs) does not supply any Vitamin B 12. Patients following such a diet should be advised to take oral Vitamin B 12 regularly. The need for Vitamin B 12 is increased by pregnancy and lactation. Deficiency has been recognized in infants of vegetarian mothers who were breast fed, even though the mothers had no symptoms of deficiency at the time.
Laboratory Tests
During the initial treatment of patients with pernicious anemia, serum potassium must be observed closely the first 48 hours and potassium replaced if necessary.
Hematocrit, reticulocyte count, Vitamin B 12, folate and iron levels should be obtained prior to treatment. Hematocrit and reticulocyte counts should be repeated daily from the 5th to 7th days of therapy and then frequently until the hematocrit is normal. If folate levels are low, folic acid should also be administered. If reticulocytes have not increased after treatment or if reticulocyte counts do not continue at least twice normal as long as the hematocrit is less than 35%, diagnosis or treatment should be reevaluated. Repeat determinations of iron and folic acid may reveal a complicating illness that might inhibit the response of the marrow.
Patients with pernicious anemia have about three times the incidence of carcinoma of the stomach as the general population, so appropriate tests for this condition should be carried out when indicated.
Drug/Laboratory Test Interactions
Persons taking most antibiotics, methotrexate and pyrimethamine invalidate folic acid and Vitamin B 12 diagnostic blood assays.
Colchicine, para-aminosalicylic acid and heavy alcohol intake for longer than two weeks may produce malabsorption of Vitamin B 12.
Carcinogenesis, Mutagenesis
Long-term studies in animals to evaluate carcinogenic potential have not been done. There is no evidence from long-term use in patients with pernicious anemia that cyanocobalamin is carcinogenic. Pernicious anemia is associated with an increased incidence of carcinoma of the stomach, but this is believed to be related to the underlying pathology and not to treatment with cyanocobalamin.
Pregnancy
Pregnancy Category C—Adequate and well-controlled studies have not been done in pregnant women. However, Vitamin B 12 is an essential vitamin and requirements are increased during pregnancy. Amounts of Vitamin B 12 that are recommended by the Food and Nutrition Board, National Academy of Science-National Research Council for pregnant women (4 mcg daily) should be consumed during pregnancy.
-
ADVERSE REACTIONS
Generalized
Anaphylactic shock and death have been reported with administration of parenteral Vitamin B 12 (see WARNINGS).
- OVERDOSAGE
-
DOSAGE AND ADMINISTRATION
Avoid using the intravenous route. Use of this product intravenously will result in almost all of the vitamin being lost in the urine.
Pernicious Anemia
Parenteral Vitamin B 12 is the recommended treatment and will be required for the remainder of the patient’s life. The oral form is not dependable. A dose of 100 mcg daily for six or seven days should be administered by intramuscular or deep subcutaneous injection. If there is clinical improvement and if a reticulocyte response is observed, the same amount may be given on alternate days for seven doses, then every three to four days for another two to three weeks. By this time hematologic values should have become normal. This regimen should be followed by 100 mcg monthly for life. Folic acid should be administered concomitantly if needed.
Patients With Normal Intestinal Absorption
Where the oral route is not deemed adequate, initial treatment similar to that for patients with pernicious anemia may be indicated depending on the severity of the deficiency. Chronic treatment should be with an oral B 12 preparation. If other vitamin deficiencies are present, they should be treated.
-
HOW SUPPLIED
Cyanocobalamin Injection, USP is supplied as follows:
Product Code Strength Each 4401 1,000 mcg per mL
1 mL fill in a 2 mL vialNDC 63323-044-00
1 mL Multiple Dose VialStore at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
PROTECT FROM LIGHT.
Use only if solution is clear and seal intact.
- SPL UNCLASSIFIED SECTION
-
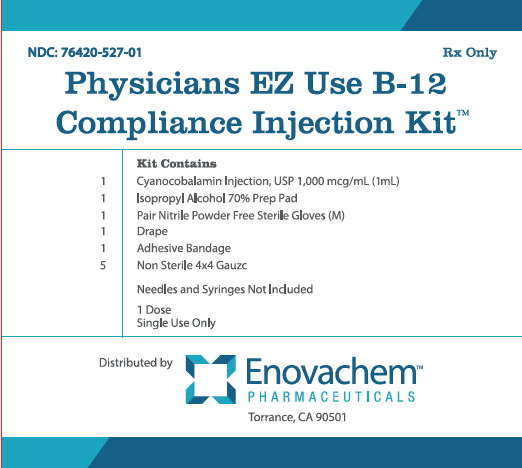
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
NDC: 76420-527-01
RX-Only
Physicians EZ Use B-12 Compliance Injection Kit™
Kit Contains
1 Cyanocobalamin Injection, USP 1,000 mcg/mL (1mL)
1 Isopropyl Alcohol 70% Prep Pad
1 Pair Nitrile Powder Free Sterile Gloves (M)
1 Drape
1 Adhesive Bandage
5 Non Sterile 4x4 Gauze
Needles and Syringes Not Included
1 Dose
Single Use Only
Distributed by:
Enovachem™
PHARMACEUTICALS
Torrance, CA 90501
-
INGREDIENTS AND APPEARANCE
PHYSICIANS EZ USE B-12 COMPLIANCE
physicians ez use b-12 compliance kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:76420-527 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76420-527-01 1 in 1 CARTON; Type 1: Convenience Kit of Co-Package 05/23/2016 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, MULTI-DOSE 1 mL Part 2 1 POUCH 5 mL Part 1 of 2 CYANOCOBALAMIN
cyanocobalamin injection, solutionProduct Information Item Code (Source) NDC:63323-044 Route of Administration INTRAMUSCULAR, SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CYANOCOBALAMIN (UNII: P6YC3EG204) (CYANOCOBALAMIN - UNII:P6YC3EG204) CYANOCOBALAMIN 1000 ug in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) BENZYL ALCOHOL (UNII: LKG8494WBH) SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63323-044-00 1 mL in 1 VIAL, MULTI-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA080557 10/18/2000 Part 2 of 2 ISOPROPYL ALCOHOL
isopropyl alcohol swabProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) (ISOPROPYL ALCOHOL - UNII:ND2M416302) ISOPROPYL ALCOHOL 70 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 5 mL in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 01/01/2007 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 10/18/2000 Labeler - Asclemed USA, Inc. (059888437)