Label: GUARDIAN VAGINAL- benzalkonium chloride douche
-
Contains inactivated NDC Code(s)
NDC Code(s): 68915-280-01, 68915-280-02 - Packager: Eastern Century Pharmaceuticals
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated September 2, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
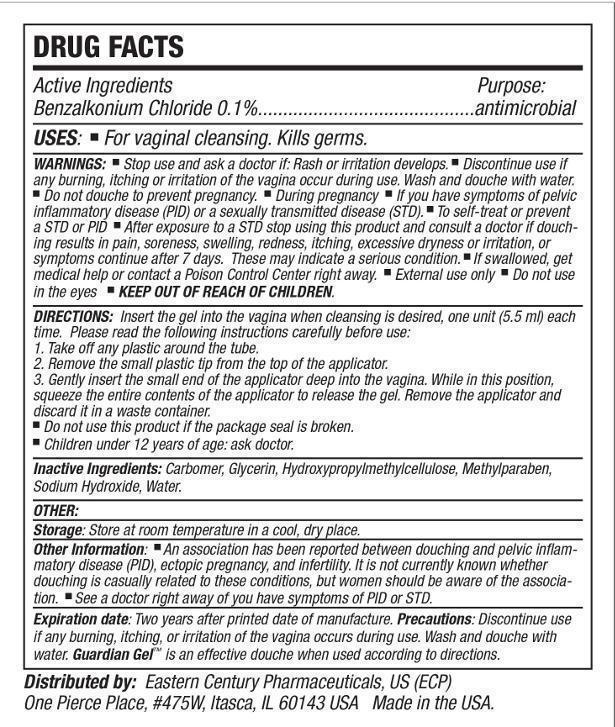
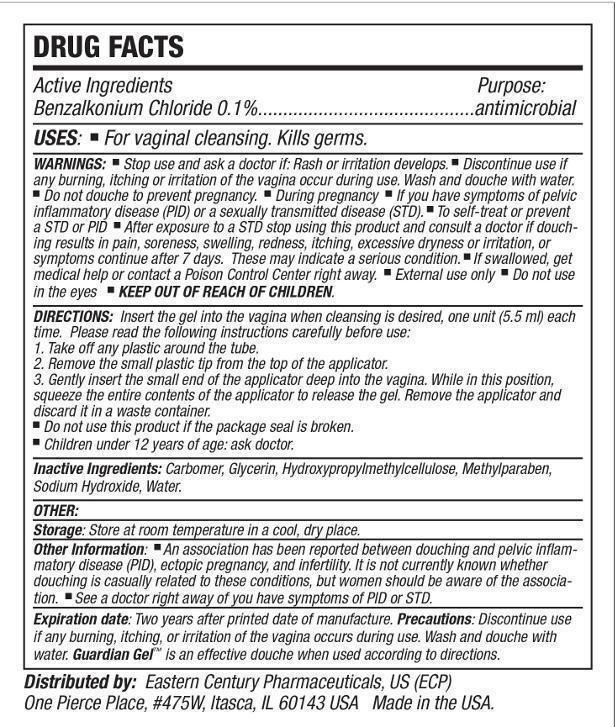
- Active ingredient
- Purpose
- Uses:
-
Warnings:
Wash and douche with water. Do not Douche to prevent pregnancy. During pregnancy. If you have symptoms of pelvic inflammatory disease (PID) or a sexually transmitted disease (STD). To self-treat or prevent a STD or PID. After exposure to a STD Stop using this product stop using this product and consult a doctor if douching results in pain, soreness, swelling, redness, itching excessive dryness or irritation, or symptoms continue after 7 days. These may indicate serious condition. If swallowed, get medical help or contact a Poison Control Center right away.
External use only.
Do not use in eyes. -
Directions:
Insert the gel into the vagina when cleansing is desired, one unit (5.5ml) each time.
Please read the following instructions carefully before use.
1. Take off any plastic around the tube.
2. Remove the small plastic tip from the top of the applicator.
3. Gently insert the small end of the applicator deep into the vagina. While in this position, squeeze the entire contents of the applicator to release the gel. Remove the applicator and discard it in a waste container.- Do not use this product if the package seal is broken.
- Children under 12 years of age: ask doctor.
- Inactive ingredients:
- Storage:
-
Other Information:
An association has been reported between douching and pelvic inflammatory disease (PID), ectopic pregnancy, and infertility. It is not currently known whether douching is casually related to these conditions, but women should be aware of this association. See a doctor right away if you have symptoms of PID or STD.
- Expiration Date:
- Precautions:
- Distributed by:
- Front Label
- Back Label
-
INGREDIENTS AND APPEARANCE
GUARDIAN VAGINAL
benzalkonium chloride doucheProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68915-280 Route of Administration VAGINAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 1 mg in 1 mL Inactive Ingredients Ingredient Name Strength CARBOMER 934 (UNII: Z135WT9208) GLYCERIN (UNII: PDC6A3C0OX) HYPROMELLOSES (UNII: 3NXW29V3WO) METHYLPARABEN (UNII: A2I8C7HI9T) SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68915-280-02 1 in 1 BOX 05/23/2011 1 NDC:68915-280-01 5.5 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 05/23/2011 Labeler - Eastern Century Pharmaceuticals (966499373) Registrant - Eastern Century Pharmaceuticals (966499373) Establishment Name Address ID/FEI Business Operations Woodbine Products Company 004220323 manufacture(68915-280)