CEDERROTH EMERGENCY EYE WASH- water liquid
Orkla Care AB
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
CEDERROTH Emergency Eye Wash
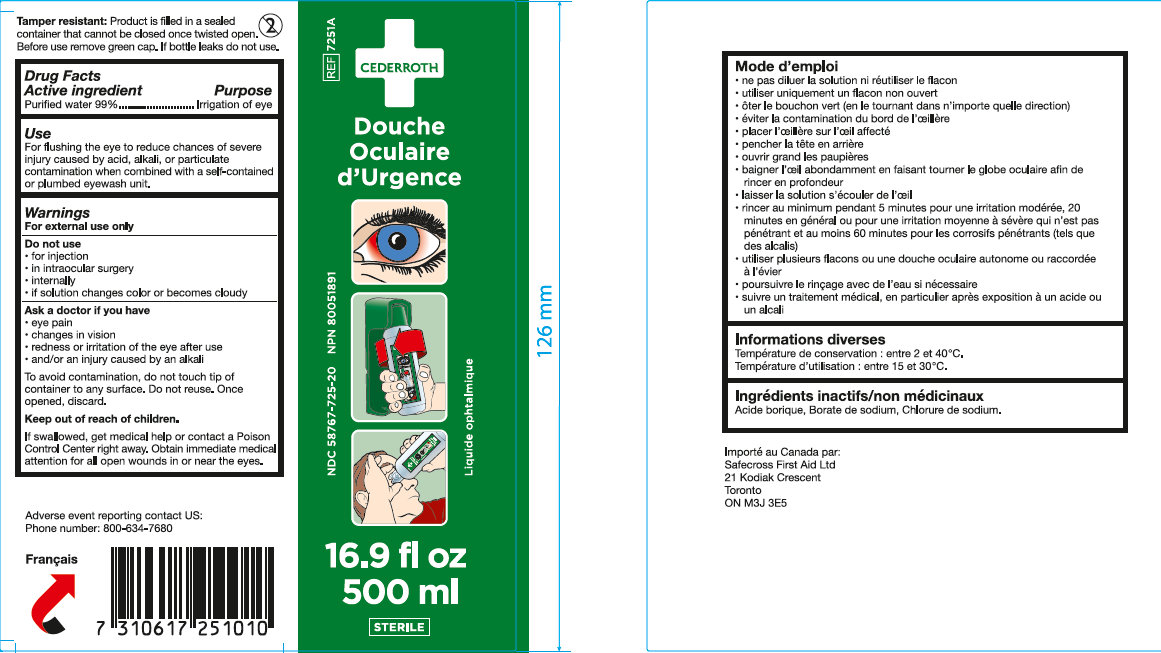
Use
For flushing the eye to reduce chances of severe injury caused by acid, alkali, or particulate contamination.
Warnings
For External Use only
Do not use
• for injection • in intraocular surgery • internally • if solution changes color or become cloudy
Directions
• do not dilute solution or reuse bottle • use only unopened bottle • remove green cap (by twisting in either direction) • avoid contamination of rim of eyecup • hold the eye cup to the eye • tilt head backward • open eyelids wide • thoroughly bathe eye with solution and rotate eyeball for thorough washing • allow solution to flow away from eye • flush affected area for a minimum of 20 minutes • continue flushing with water if necessary • obtain medical treatment, especially after exposure to acid or alkali.
| CEDERROTH EMERGENCY EYE WASH
water liquid |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Orkla Care AB (354061566) |
| Registrant - Orkla Care AB (354061566) |