VP-ZEL- niacinamide, pyridoxine, folic acid, zinc oxide, cupric oxide, and azelaic acid tablet
Virtus Pharmaceuticals
----------
VP-Zel Tabs
DESCRIPTION
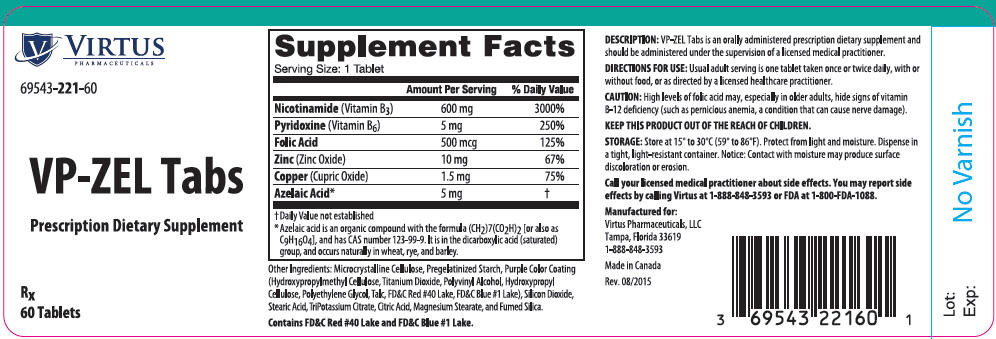
VP-ZEL Tabs is an orally administered prescription dietary supplement specifically formulated for the dietary management of patients requiring increased folate levels and nutritional supplementation in addition to their dietary intake.
VP-ZEL Tabs should be administered under the supervision of a licensed medical practitioner.
DIRECTIONS FOR USE
Usual adult serving is one tablet taken once or twice a day, with or without food, or as directed by a licensed medical practitioner.
Folate therapy alone is inadequate for treatment of a vitamin B12 deficiency.
Not for use by pregnant or nursing women. Not for use by children.
| Supplement Facts | ||
|---|---|---|
| Serving Size: 1 Tablet | ||
| Amount Per Serving | % Daily Value | |
|
Nicotinamide (Vitamin B3) |
600 mg |
3000% |
|
Pyridoxine (Vitamin B6) |
5 mg |
250% |
|
Folic Acid |
500 mcg |
125% |
|
Zinc (Zinc Oxide) |
10 mg |
67% |
|
Copper (Cupric Oxide) |
1.5 mg |
75% |
|
Azelaic Acid* |
5 mg | |
Other Ingredients: Microcrystalline Cellulose, Pregelatinized Starch, Purple Color Coating (Hydroxypropylmethyl Cellulose, Titanium Dioxide, Polyvinyl Alcohol, Hydroxypropyl Cellulose, Polyethylene Glycol, Talc, FD&C Red #40 Lake, FD&C Blue #1 Lake), Silicon Dioxide, Stearic Acid, TriPotassium Citrate, Citric Acid, Magnesium Stearate, and Fumed Silica.
Contains FD&C Red #40 Lake and FD&C Blue #1 Lake.
KEEP THIS PRODUCT OUT OF THE REACH OF CHILDREN.
CONTRAINDICATIONS
This product is contraindicated in patients with a known hypersensitivity to any of its ingredients.
CAUTION
High levels of folic acid may, especially in older adults, hide signs of vitamin B-12 deficiency (such as pernicious anemia, a condition that can cause nerve damage).
High levels of niacin can potentiate hepatotoxicity in patients taking known hepatotoxins: leflunomide, teriflunomide, lomitapide, mipomersen, and statins (lovastatin, simvastatin, atorvastatin, cerivastatin, pitavastatin, rosuvastatin, and red yeast rice).
SIDE EFFECTS
Allergic sensitization has been reported following oral administration of folic acid. Paresthesia, somnolence, nausea and headaches have been reported with pyridoxine hydrochloride (vitamin B6).
Flushing may occur after oral administration of nicotinamide. Nonspecific gastrointestinal side effects also may occur.
DRUG INTERACTIONS
Drugs which may interact with folate include:
- •
- Antiepileptic drugs (AED): The AED class including, but not limited to, phenytoin, carbamazepine, primidone, valproic acid, fosphenytoin, valproate, phenobarbital and lamotrigine have been shown to impair folate absorption and increase the metabolism of circulating folate.
- •
- Additionally, concurrent use of folic acid has been associated with enhanced phenytoin metabolism, lowering the level of the AED in the blood and allowing breakthrough seizures to occur. Caution should be used when prescribing this product among patients who are receiving treatment with phenytoin or other anticonvulsants.
- •
- Capecitabine: Folinic acid (5-formyltetrahydrofolate) may increase the toxicity of Capecitabine.
- •
- Cholestyramine: Reduces folic acid absorption and reduces serum folate levels.
- •
- Colestipol: Reduces folic acid absorption and reduces serum folate levels.
- •
- Cycloserine: Reduces folic acid absorption and reduces serum folate levels.
- •
- Dihydrofolate Reductase Inhibitors (DHFRI): DHFRIs block the conversion of folic acid to its active forms, and lower plasma and red blood cell folate levels. DHFRIs include aminopterin, methotrexate, pyrimethamine, triamterene, and trimethoprim.
- •
- Fluoxetine: Fluoxetine exerts a noncompetitive inhibition of the 5-methyltetrahydrofolate active transport in the intestine.
- •
- Isotretinoin: Reduced folate levels have occurred in some patients taking isotretinoin.
- •
- L-dopa, triamterene, colchicine, and trimethoprim may decrease plasma folate levels.
- •
- Nonsteroidal Anti-inflammatory Drugs (NSAIDs): NSAIDs have been shown to inhibit some folate dependent enzymes in laboratory experiments. NSAIDs include ibuprofen, naproxen, indomethacin and sulindac.
- •
- Oral Contraceptives: Serum folate levels may be depressed by oral contraceptive therapy.
- •
- Methylprednisolone: Reduced serum folate levels have been noted after treatment with methylprednisolone.
- •
- Pancreatic Enzymes: Reduced folate levels have occurred in some patients taking pancreatic extracts, such as pancreatin and pancrelipase.
- •
- Pentamidine: Reduced folate levels have been seen with prolonged intravenous pentamidine.
- •
- Pyrimethamine: High levels of folic acid may result in decreased serum levels of pyrimethamine.
- •
- Smoking and Alcohol: Reduced serum folate levels have been noted.
- •
- Sulfasalazine: Inhibits the absorption and metabolism of folic acid.
- •
- Metformin treatment in patients with type 2 diabetes decreases serum folate.
- •
- Warfarin can produce significant impairment in folate status after a 6-month therapy.
Drugs which may interact with nicotinamide:
- •
- The clearance of primidone and carbamazepine may be reduced with the concomitant use of nicotinamide.
- •
- Niacin can potentiate the hepatotoxicity of statins and other hepatotoxic drugs (see Cautions).
Drugs which may interact with zinc oxide:
- •
- The absorption of quinolones or tetracycline may be decreased with the concomitant use of zinc.
Drugs which may interact with cupric oxide:
- •
- Concomitant use of penicillamine and copper can cause decreased absorption of both substances.
HOW SUPPLIED
VP-ZEL Tabs are supplied as coated, purple colored, oval-shaped tablets debossed on one side with "V221", and are supplied in bottles of 60 tablets.
| VP-ZEL
niacinamide, pyridoxine, folic acid, zinc oxide, cupric oxide, and azelaic acid tablet |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| Supplement Facts | ||
| Serving Size : | Serving per Container : | |
| Amount Per Serving | % Daily Value | |
|---|---|---|
| color | ||
| size (solid drugs) | 18 mm | |
| shape | ||
| scoring | 1 | |
| imprint | ||
| Labeler - Virtus Pharmaceuticals (079659493) |