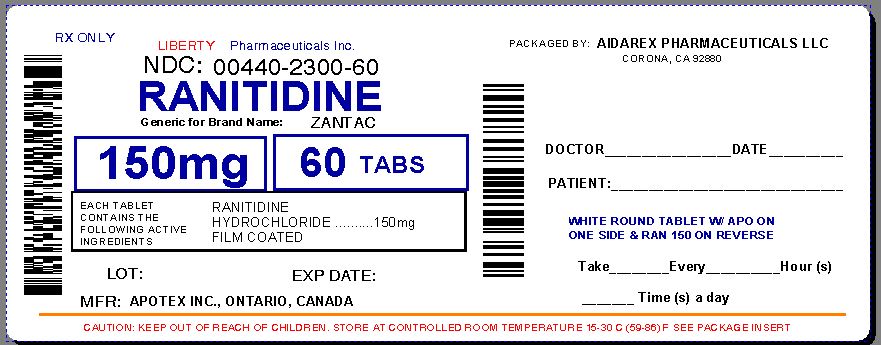
Label: RANITIDINE- ranitidine hydrochloride tablet, film coated
-
Contains inactivated NDC Code(s)
NDC Code(s): 0440-2300-60 - Packager: Liberty Pharmaceuticals, Inc.
- This is a repackaged label.
- Source NDC Code(s): 60505-2880
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated September 24, 2013
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient(s)
- Purpose
- Use(s)
-
Warnings
Allergy alert: Do not use if you are allergic to ranitidine or any other acid reducers.
Do not use
- with other acid reducers
- if you have kidney disease, except under the advice and supervision of a doctor
- if you have trouble or pain swallowing food, vomiting with blood, or bloody or black stools. These may be signs of a serious condition. See your doctor
Ask a doctor before use if you have
- nausea or vomiting
- stomach pain
- unexplained weight loss
- frequent chest pain
- frequent wheezing, particularly with heartburn
- had heartburn over 3 months. This may be a sign of a more serious condition.
- heartburn with lightheadedness, sweating or dizziness
- chest pain or shoulder pain with shortness of breath; sweating; pain spreading to arms, neck or shoulders; or lightheadedness
-
Directions
Adults and Children 12 years and over:
- to relieve symptoms, swallow 1 tablet with a glass of water
- to prevent symptoms, swallow 1 tablet with a glass of water 30 to 60 minutes before eating food or drinking beverages that cause heartburn
- can be used up to twice daily (do not take more than 2 tablets in 24 hours)
- do not chew tablet
- children under 12 years: ask a doctor
- Other Information
- Inactive Ingredients
-
Questions or Comments?
Call 1-800-706-5575 (Monday to Friday 8:30 a.m. – 5:00 p.m. Eastern Standard Time)
Consumer Information
What you should know about
MAXIMUM STRENGTH
Ranitidine Tablets USP, 150 mg / Acid Reducer
(Please read all this information before taking MAXIMUM STRENGTH Ranitidine Tablets USP, 150 mg. Save this leaflet for future reference)
What are MAXIMUM STRENGTH Ranitidine Tablets USP, 150 mg?
MAXIMUM STRENGTH Ranitidine Tablets USP, 150 mg contains 150 mg of ranitidine (as ranitidine hydrochloride, 168 mg), a medicine that doctors have prescribed more than 200 million times worldwide.
What symptoms do MAXIMUM STRENGTH Ranitidine Tablets USP, 150 mg treat and prevent?
MAXIMUM STRENGTH Ranitidine Tablets USP, 150 mg relieves and prevents heartburn associated with acid indigestion and sour stomach.
Certain foods or beverages, and even lying down to sleep, can cause heartburn associated with acid indigestion and sour stomach. It is normal for the stomach to produce acid, especially after consuming food or beverages. However, acid in the wrong place, such as the esophagus, or too much acid, can cause burning pain and discomfort.
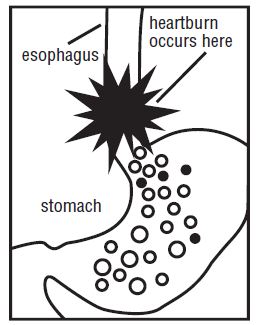
Excellent Safety Record
- The ingredient in MAXIMUM STRENGTH Ranitidine Tablets USP, 150 mg, ranitidine, has been prescribed by doctors for years to treat millions of patients safely and effectively. The active ingredient in MAXIMUM STRENGTH Ranitidine Tablets USP, 150 mg has been taken safely with many frequently prescribed medications.
- MAXIMUM STRENGTH Ranitidine Tablets USP, 150 mg are sodium and sugar free.
How should I take MAXIMUM STRENGTH Ranitidine Tablets USP, 150 mg?
- To relieve symptoms, swallow 1 tablet with a glass of water.
- To prevent symptoms, swallow 1 tablet with a glass of water 30 to 60 minutes before eating food or drinking beverages that cause heartburn.
This medicine can be used up to twice daily (do not take more than 2 tablets in 24 hours).
MAXIMUM STRENGTH Ranitidine Tablets USP, 150 mg should not be given to children under 12 years old unless directed by a doctor.
How do MAXIMUM STRENGTH Ranitidine Tablets USP, 150 mg work?
MAXIMUM STRENGTH Ranitidine Tablets USP, 150 mg reduces the production of stomach acid. This is what makes MAXIMUM STRENGTH Ranitidine Tablets USP, 150 mg different from antacids, which neutralize the acid already in your stomach. Antacids do not reduce the production of acid.
Tips for managing heartburn
- Do not lie flat or bend over soon after eating
- Do not eat late at night, or just before bedtime
- Certain foods or drinks are more likely to cause heartburn, such as rich, spicy, fatty, and fried foods, chocolate, caffeine, alcohol, even some fruits and vegetables
- Eat slowly and do not eat big meals
- If you are overweight, lose weight
- If you smoke, quit smoking
- Raise the head of your bed
- Wear loose fitting clothing around your stomach
- Allergy alert: Do not use if you are allergic to ranitidine or other acid reducers
When should I see a doctor?
-
Do not use
- with other acid reducers
- if you have kidney disease, except under the advice and supervision of a doctor
- if you have trouble or pain swallowing food, vomiting with blood, or bloody or black stools. These may be signs of a serious condition. See your doctor.
-
Ask a doctor before use if you have
- nausea or vomiting
- stomach pain
- unexplained weight loss
- frequent chest pain
- frequent wheezing, particularly with heartburn
- had heartburn over 3 months. This may be a sign of a more serious condition
- heartburn with lightheadedness, sweating or dizziness
- chest pain or shoulder pain with shortness of breath; sweating; pain spreading to arms, neck or shoulders; or lightheadedness
-
Stop use and ask a doctor if
- your heartburn continues or worsens
- you need to take this product for more than 14 days
- If pregnant or breast feeding, ask a health professional before use.
- Keep out of reach of children. In case of overdose get medical help or contact a Poison Control Center right away.
Questions or Comments?
Call 1-800-706-5575 (Monday to Friday, 8:30 am – 5:00 pm Eastern Standard Time)
Manufactured by: Manufactured for:
Apotex Inc. Apotex Corp.
Toronto, Ontario Weston, FL
Canada M9L 1T9 33326
Repackaged By :
Aidarex Pharmaceuticals LLC,
Corona, CA 92880 - Principal Display Panel
-
INGREDIENTS AND APPEARANCE
RANITIDINE
ranitidine hydrochloride tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0440-2300(NDC:60505-2880) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength RANITIDINE HYDROCHLORIDE (UNII: BK76465IHM) (RANITIDINE - UNII:884KT10YB7) RANITIDINE 150 mg Inactive Ingredients Ingredient Name Strength CARNAUBA WAX (UNII: R12CBM0EIZ) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HYPROMELLOSES (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POLYDEXTROSE (UNII: VH2XOU12IE) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) VANILLIN (UNII: CHI530446X) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) Product Characteristics Color PINK Score no score Shape ROUND Size 9mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0440-2300-60 60 in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA200172 07/15/2013 Labeler - Liberty Pharmaceuticals, Inc. (012568840)