AUROTUSSIN MUCUS PLUS CHEST CONGESTION (ORIGINAL) - guaifenesin liquid
Aurohealth LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
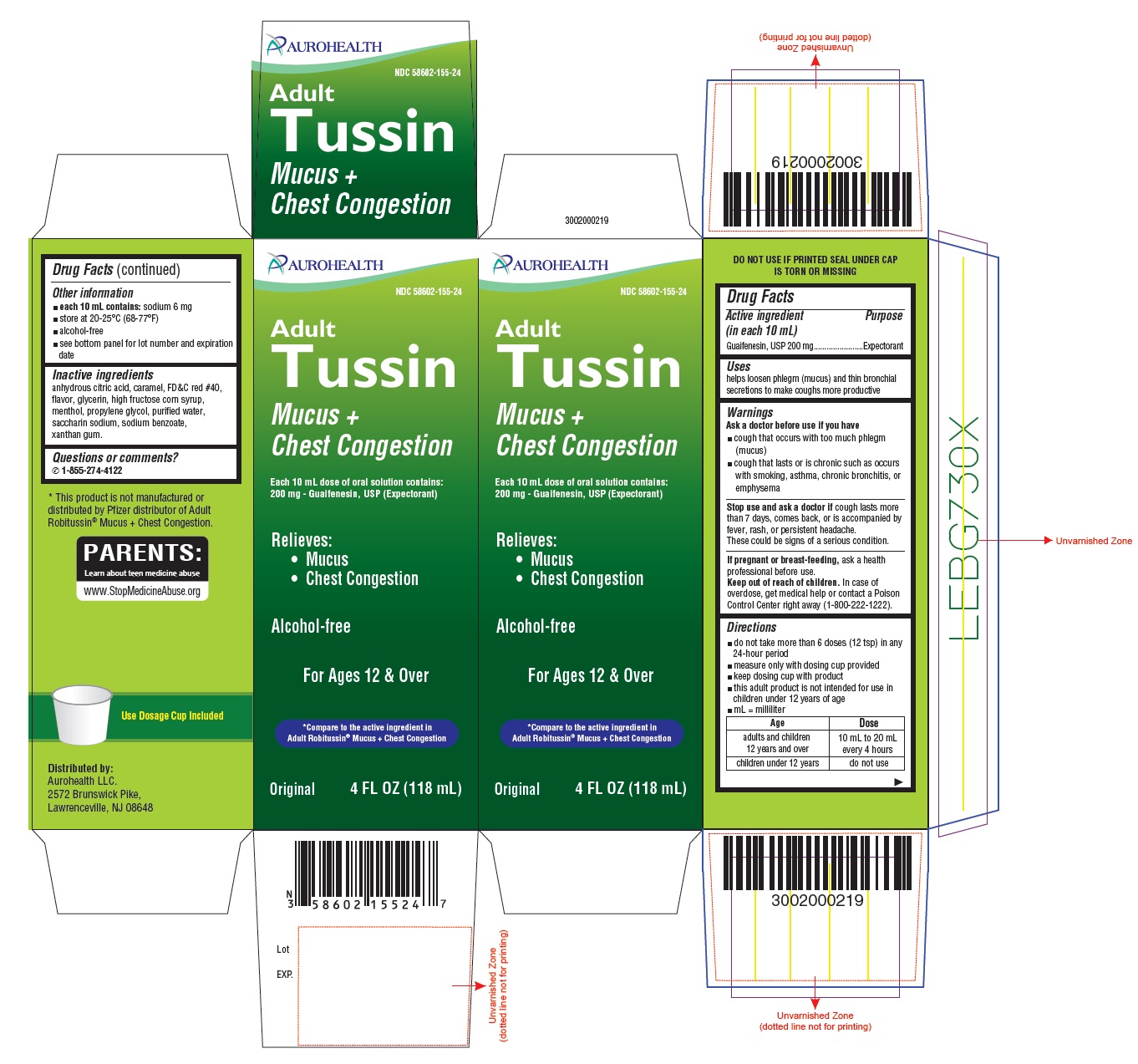
Drug Facts
Warnings
Ask a doctor before use if you have
- cough that occurs with too much phlegm (mucus)
- cough that lasts or is chronic such as occurs with smoking, asthma, chronic bronchitis, or emphysema
Stop use and ask a doctor if
cough lasts more than 7 days, comes back, or is accompanied by fever, rash, or persistent headache. These could be signs of a serious condition.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away (1-800-222-1222).
Directions
- do not take more than 6 doses (12 tsp) in any 24-hour period
- measure only with dosing cup provided
- keep dosing cup with product
- this adult product is not intended for use in children under 12 years of age
- mL = milliliter
| Age
| Dose
|
| adults and children 12 years and over | 10 mL to 20 mL every 4 hours |
| children under 12 years | do not use |
Inactive ingredients
anhydrous citric acid, caramel, FD&C red #40, flavor, glycerin, high fructose corn syrup, menthol, propylene glycol, purified water, saccharin sodium, sodium benzoate, xanthan gum.
Questions or Comments
1-855-274-4122
Distributed by:
Aurohealth LLC.
2572 Brunswick Pike,
Lawrenceville, NJ 08648
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL -4 FL OZ (118 mL Bottle)
AUROHEALTH
NDC 58602-155-24
Adult
Tussin
Mucus +
Chest Congesion
Each 10 mL dose of oral solution contains:
200 mg - Guaifenesin, USP (Expectorant)
Relieves:
Mucus
Chest Congestion
Alcohol-free
For Ages 12 & Over
Compare to the active ingredient in
Adult Robitussin® Mucus+ Chest Congestion
Original 4 FL OZ (118 mL)
| AUROTUSSIN MUCUS PLUS CHEST CONGESTION (ORIGINAL)
guaifenesin liquid |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Aurohealth LLC (078728447) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Aurohealth LLC | 078728447 | MANUFACTURE(58602-155) | |