Label: CEFUROXIME AXETIL FOR ORAL SUSPENSION- cefuroxime axetil suspension
-
Contains inactivated NDC Code(s)
NDC Code(s): 63304-963-03, 63304-963-04, 63304-964-03, 63304-964-04 - Packager: Ranbaxy Pharmaceuticals Inc
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated August 19, 2013
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTION
To reduce the development of drug-resistant bacteria and maintain the effectiveness of cefuroxime axetil for oral suspension and other antibacterial drugs, cefuroxime axetil for oral suspension should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria.
-
DESCRIPTION
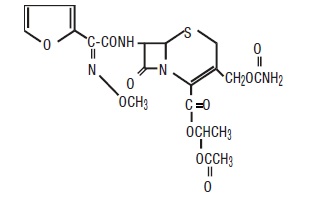
Cefuroxime axetil for oral suspension, USP contains cefuroxime as cefuroxime axetil. Cefuroxime axetil USP is a semisynthetic, broad-spectrum cephalosporin antibiotic for oral administration.
Chemically, cefuroxime axetil, the 1-(acetyloxy) ethyl ester of cefuroxime, is (RS)-1- hydroxyethyl (6R,7R)-7-[2-(2-furyl)glyoxylamido]-3-(hydroxymethyl)-8-oxo-5-thia-1- azabicyclo[4.2.0]oct-2-ene-2-carboxylate, 72-(Z)-(O-methyl-oxime), 1-acetate 3-carbamate. Its molecular formula is C20H22N4O10S, and it has a molecular weight of 510.48.
Cefuroxime axetil is in the crystalline form and has the following structural formula:
Cefuroxime axetil for oral suspension USP, when reconstituted with water, provides the equivalent of 125 mg or 250 mg of cefuroxime (as cefuroxime axetil) per 5 mL of suspension. Cefuroxime axetil USP for oral suspension contains the following inactive ingredients: aspartame, hypromellose phthalate, mannitol, methacrylic acid copolymer, monosodium citrate, peppermint flavor, silicon dioxide, sodium benzoate, sodium chloride, sucrose, tutti frutti flavor, xanthan gum.
-
CLINICAL PHARMACOLOGY
Absorption and Metabolism: After oral administration, cefuroxime axetil is absorbed from the gastrointestinal tract and rapidly hydrolyzed by nonspecific esterases in the intestinal mucosa and blood to cefuroxime. Cefuroxime is subsequently distributed throughout the extracellular fluids. The axetil moiety is metabolized to acetaldehyde and acetic acid.
Pharmacokinetics: Approximately 50% of serum cefuroxime is bound to protein. Serum pharmacokinetic parameters for cefuroxime axetil for oral suspension are shown in Table 1.
Table 1. Postprandial Pharmacokinetics of Cefuroxime Administered as Cefuroxime Axetil for Oral Suspension to Pediatric Patients* Dose† (Cefuroxime Equivalent) n Peak Plasma Concentration (mcg/mL) Time of Peak Plasma Concentration (hr) Mean Elimination Half-Life (hr) AUC (mcg-hr mL) 10 mg/kg 8 3.3 3.6 1.4 12.4 15 mg/kg 12 5.1 2.7 1.9 22.5 20 mg/kg 8 7 3.1 1.9 32.8 †Drug administered with milk or milk products
Comparative Pharmacokinetic Properties: A 250 mg/5 mL dose of cefuroxime axetil for oral suspension is bioequivalent to 2 times 125 mg/5 mL dose of cefuroxime axetil for oral suspension when administered with food (see Table 2). Cefuroxime axetil for oral suspension was not bioequivalent to cefuroxime axetil tablets when tested in healthy adults. The tablet and powder for oral suspension formulations are NOT substitutable on a milligram-per-milligram basis. The area under the curve for the suspension averaged 91% of that for the tablet, and the peak plasma concentration for the suspension averaged 71% of the peak plasma concentration of the tablets. Therefore, the safety and effectiveness of both the tablet and oral suspension formulations had to be established in separate clinical trials.
Table 2: Pharmacokinetics of Cefuroxime Administered as 250 mg/5 mL or 2 x 125 mg/5 mL Cefuroxime Axetil for Oral Suspension to Adults* With Food Dose (Cefuroxime Equivalent) Peak Plasma Concentration (mcg/mL) Time of Peak Plasma Concentration (hr) Mean Elimination Half-Life (hr) AUC (mcg-hr mL) 250 mg/5 mL 2.23 3 1.40 8.92 2 x 125 mg/5 mL 2.37 3 1.44 9.75 *Mean values of 18 healthy adult volunteers
Food Effect on Pharmacokinetics: All pharmacokinetic and clinical effectiveness and safety studies in pediatric patients using the suspension formulation were conducted in the fed state. No data are available on the absorption kinetics of the suspension formulation when administered to fasted pediatric patients.
Renal Excretion: Cefuroxime is excreted unchanged in the urine; in adults, approximately 50% of the administered dose is recovered in the urine within 12 hours. The pharmacokinetics of cefuroxime in the urine of pediatric patients have not been studied at this time. Until further data are available, the renal pharmacokinetic properties of cefuroxime axetil established in adults should not be extrapolated to pediatric patients.
Because cefuroxime is renally excreted, the serum half-life is prolonged in patients with reduced renal function. In a study of 20 elderly patients (mean age = 83.9 years) having a mean creatinine clearance of 34.9 mL/min, the mean serum elimination half-life was 3.5 hours. Despite the lower elimination of cefuroxime in geriatric patients, dosage adjustment based on age is not necessary (see PRECAUTIONS: Geriatric Use).
Microbiology: The in vivo bactericidal activity of cefuroxime axetil is due to cefuroxime's binding to essential target proteins and the resultant inhibition of cell-wall synthesis.
Cefuroxime has bactericidal activity against a wide range of common pathogens, including many beta-lactamase-producing strains. Cefuroxime is stable to many bacterial beta-lactamases, especially plasmid-mediated enzymes that are commonly found in enterobacteriaceae.
Cefuroxime has been demonstrated to be active against most strains of the following microorganisms both in vitro and in clinical infections as described in the INDICATIONS AND USAGE section (see INDICATIONS AND USAGE section).
Aerobic Gram-Positive Microorganisms:
Staphylococcus aureus (including beta-lactamase-producing strains)
Aerobic Gram-Negative Microorganisms:
Haemophilus influenzae (including beta-lactamase-producing strains)
Moraxella catarrhalis (including beta-lactamase-producing strains)
Neisseria gonorrhoeae (including beta-lactamase-producing strains)
Cefuroxime has been shown to be active in vitro against most strains of the following microorganisms; however, the clinical significance of these findings is unknown.
Cefuroxime exhibits in vitro minimum inhibitory concentrations (MICs) of 4 mcg/mL or less (systemic susceptible breakpoint) against most (> 90%) strains of the following microorganisms; however, the safety and effectiveness of cefuroxime in treating clinical infections due to these microorganisms have not been established in adequate and well-controlled trials.
Aerobic Gram-Positive Microorganisms:
NOTE: Listeria monocytogenes and certain strains of enterococci, e.g., Enterococcus faecalis (formerly Streptococcus faecalis), are resistant to cefuroxime. Methicillin-resistant staphylococci are resistant to cefuroxime.
Aerobic Gram-Negative Microorganisms:
NOTE: Pseudomonas spp., Campylobacter spp., Acinetobactercalcoaceticus, Legionella spp., and most strains of Serratia spp. and Proteus vulgaris are resistant to most first- and second-generation cephalosporins. Some strains of Morganellamorganii, Enterobactercloacae, and Citrobacter spp. have been shown by in vitro tests to be resistant to cefuroxime and other cephalosporins.
NOTE: Most strains of Clostridium difficile and Bacteroides fragilis are resistant to cefuroxime.
SusceptibilityTests: DilutionTechniques: Quantitative methods that are used to determine MICs provide reproducible estimates of the susceptibility of bacteria to antimicrobial compounds. One such standardized procedure uses a standardized dilution method1 (broth, agar, or microdilution) or equivalent with cefuroxime powder. The MIC values obtained should be interpreted according to the following criteria:
MIC (mcg/mL) Interpretation ≤ 4 (S) Susceptible 8 to16 (I) Intermediate ≥ 32 (R) Resistant A report of "Susceptible" indicates that the pathogen, if in the blood, is likely to be inhibited by usually achievable concentrations of the antimicrobial compound in blood. A report of "Intermediate" indicates that inhibitory concentrations of the antibiotic may be achieved if high dosage is used or if the infection is confined to tissues or fluids in which high antibiotic concentrations are attained. This category also provides a buffer zone that prevents small, uncontrolled technical factors from causing major discrepancies in interpretation. A report of "Resistant" indicates that usually achievable concentrations of the antimicrobial compound in the blood are unlikely to be inhibitory and that other therapy should be selected.
Standardized susceptibility test procedures require the use of laboratory control microorganisms. Standard cefuroxime powder should give the following MIC values:
Microorganism MIC (mcg/mL) Escherichia coli ATCC 25922 2 to 8 Staphylococcus aureus ATCC 29213 0.5 to 2 DiffusionTechniques: Quantitative methods that require measurement of zone diameters provide estimates of the susceptibility of bacteria to antimicrobial compounds. One such standardized procedure2 that has been recommended (for use with disks) to test the susceptibility of microorganisms to cefuroxime uses the 30 mcg cefuroxime disk. Interpretation involves correlation of the diameter obtained in the disk test with the MIC for cefuroxime.
Reports from the laboratory providing results of the standard single-disk susceptibility test with a 30 mcg cefuroxime disk should be interpreted according to the following criteria:
Zone Diameter (mm) Interpretation ≥ 23 (S) Susceptible 15 to 22 (I) Intermediate ≤ 14 (R) Resistant Interpretation should be as stated above for results using dilution techniques.
As with standard dilution techniques, diffusion methods require the use of laboratory control microorganisms. The 30 mcg cefuroxime disk provides the following zone diameters in these laboratory test quality control strains:
Microorganism Zone Diameter (mm) Escherichia coli ATCC 25922 20 to 26 Staphylococcus aureus ATCC 25923 27 to 35 -
INDICATIONS AND USAGE
NOTE: CEFUROXIME AXETIL TABLETS AND CEFUROXIME AXETIL FOR ORAL SUSPENSION ARE NOT BIOEQUIVALENT AND ARE NOT SUBSTITUTABLE ON AMILLIGRAM-PER-MILLIGRAM BASIS (SEE CLINICAL PHARMACOLOGY).
Cefuroxime axetil for oral suspension is indicated for the treatment of pediatric patients 3 months to 12 years of age with mild to moderate infections caused by susceptible strains of the designated microorganisms in the conditions listed below. The safety and effectiveness of cefuroxime axetil for oral suspension in the treatment of infections other than those specifically listed below have not been established either by adequate and well-controlled trials or by pharmacokinetic data with which to determine an effective and safe dosing regimen.
- Pharyngitis/Tonsillitis caused by Streptococcus pyogenes. NOTE: The usual drug of choice in the treatment and prevention of streptococcal infections, including the prophylaxis of rheumatic fever, is penicillin given by the intramuscular route. Cefuroxime axetil for oral suspension is generally effective in the eradication of streptococci from the nasopharynx; however, substantial data establishing the efficacy of cefuroxime in the subsequent prevention of rheumatic fever are not available. Please also note that in all clinical trials, all isolates had to be sensitive to both penicillin and cefuroxime. There are no data from adequate and well-controlled trials to demonstrate the effectiveness of cefuroxime in the treatment of penicillin-resistant strains of Streptococcus pyogenes.
- Acute Bacterial Otitis Media caused by Streptococcus pneumoniae, Haemophilus influenzae (including beta-lactamase-producing strains), Moraxella catarrhalis (including beta-lactamase-producing strains), or Streptococcus pyogenes.
- Impetigo caused by Staphylococcus aureus (including beta-lactamase-producing strains) or Streptococcus pyogenes.
To reduce the development of drug-resistant bacteria and maintain the effectiveness of cefuroxime axetil for oral suspension and other antibacterial drugs, cefuroxime axetil for oral suspension should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
- CONTRAINDICATIONS
-
WARNINGS
CEFUROXIME AXETIL TABLETS AND CEFUROXIME AXETIL FOR ORAL SUSPENSION ARE NOT BIOEQUIVALENT AND ARE THEREFORE NOT SUBSTITUTABLE ON A MILLIGRAM-PER-MILLIGRAM BASIS (SEE CLINICAL PHARMACOLOGY).
BEFORE THERAPY WITH CEFUROXIME AXETIL PRODUCTS IS INSTITUTED, CAREFUL INQUIRY SHOULD BE MADE TO DETERMINE WHETHER THE PATIENT HAS HAD PREVIOUS HYPERSENSITIVITY REACTIONS TO CEFUROXIME AXETIL PRODUCTS, OTHER CEPHALOSPORINS, PENICILLINS, OR OTHER DRUGS. IF THIS PRODUCT IS TO BE GIVEN TO PENICILLIN-SENSITIVE PATIENTS, CAUTION SHOULD BE EXERCISED BECAUSE CROSS-HYPERSENSITIVITY AMONG BETA-LACTAM ANTIBIOTICS HAS BEEN CLEARLY DOCUMENTED AND MAY OCCUR IN UP TO 10% OF PATIENTS WITH A HISTORY OF PENICILLIN ALLERGY. IF A CLINICALLY SIGNIFICANT ALLERGIC REACTION TO CEFUROXIME AXETIL PRODUCTS OCCURS, DISCONTINUE THE DRUG AND INSTITUTE APPROPRIATE THERAPY. SERIOUS ACUTE HYPERSENSITIVITY REACTIONS MAY REQUIRE TREATMENT WITH EPINEPHRINE AND OTHER EMERGENCY MEASURES, INCLUDING OXYGEN, INTRAVENOUS FLUIDS, INTRAVENOUS ANTIHISTAMINES, CORTICOSTEROIDS, PRESSOR AMINES, AND AIRWAY MANAGEMENT, AS CLINICALLY INDICATED.
Clostridium difficile associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including cefuroxime axetil for oral suspension, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
-
PRECAUTIONS
General
As with other broad-spectrum antibiotics, prolonged administration of cefuroxime axetil may result in overgrowth of nonsusceptible microorganisms. If superinfection occurs during therapy, appropriate measures should be taken.
Cephalosporins, including cefuroxime axetil, should be given with caution to patients receiving concurrent treatment with potent diuretics because these diuretics are suspected of adversely affecting renal function.
Cefuroxime axetil, as with other broad-spectrum antibiotics, should be prescribed with caution in individuals with a history of colitis. The safety and effectiveness of cefuroxime axetil have not been established in patients with gastrointestinal malabsorption. Patients with gastrointestinal malabsorption were excluded from participating in clinical trials of cefuroxime axetil.
Cephalosporins may be associated with a fall in prothrombin activity. Those at risk include patients with renal or hepatic impairment, or poor nutritional state, as well as patients receiving a protracted course of antimicrobial therapy, and patients previously stabilized on anticoagulant therapy. Prothrombin time should be monitored in patients at risk and exogenous vitamin K administered as indicated.
Prescribing cefuroxime axetil for oral suspension in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
Diarrhea is a common problem caused by antibiotics which usually ends when the antibiotic is discontinued. Sometimes after starting treatment with antibiotics, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as 2 or more months after having taken the last dose of the antibiotic. If this occurs, patients should contact their physician as soon as possible.
Information for Patients/Caregivers (Pediatric)
Phenylketonurics: Cefuroxime axetil for Oral Suspension 125 mg/5 ml and 250 mg/5 mL contain phenylalanine 4.5 mg per 5 mL (1 teaspoonful) constituted suspension.
1. During clinical trials, the tablet was tolerated by pediatric patients old enough to swallow the cefuroxime axetil tablet whole. The crushed tablet has a strong, persistent, bitter taste and should not be administered to pediatric patients in this manner. Pediatric patients who cannot swallow the tablet whole should receive the oral suspension.
2. Discontinuation of therapy due to taste and/or problems of administering this drug occurred in 1.4% of pediatric patients given the oral suspension. Complaints about taste (which may impair compliance) occurred in 5% of pediatric patients.
3. Patients should be counseled that antibacterial drugs, including cefuroxime axetil for oral suspension, should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When cefuroxime axetil for oral suspension is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may: (1) decrease the effectiveness of the immediate treatment, and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by cefuroxime axetil for oral suspension or other antibacterial drugs in the future.
Drug/Laboratory Test Interactions
A false-positive reaction for glucose in the urine may occur with copper reduction tests (Benedict's or Fehling's solution or with CLINITEST® tablets), but not with enzyme-based tests for glycosuria (e.g., CLINISTIX®). As a false-negative result may occur in the ferricyanide test, it is recommended that either the glucose oxidase or hexokinase method be used to determine blood/plasma glucose levels in patients receiving cefuroxime axetil. The presence of cefuroxime does not interfere with the assay of serum and urine creatinine by the alkaline picrate method.
Drug/Drug Interactions
Concomitant administration of probenecid with cefuroxime axetil tablets increases the area under the serum concentration versus time curve by 50%. The peak serum cefuroxime concentration after a 1.5 g single dose is greater when taken with 1 g of probenecid (mean = 14.8 mcg/mL) than without probenecid (mean = 12.2 mcg/mL).
Drugs that reduce gastric acidity may result in a lower bioavailability of cefuroxime axetil compared with that of fasting state and tend to cancel the effect of postprandial absorption.
In common with other antibiotics, cefuroxime axetil may affect the gut flora, leading to lower estrogen reabsorption and reduced efficacy of combined oral estrogen/progesterone contraceptives.
Carcinogenesis, Mutagenesis, and Impairment of Fertility
Although lifetime studies in animals have not been performed to evaluate carcinogenic potential, no mutagenic activity was found for cefuroxime axetil in a battery of bacterial mutation tests. Positive results were obtained in an in vitro chromosome aberration assay, however, negative results were found in an in vivo micronucleus test at doses up to 1.5 g/kg. Reproduction studies in rats at doses up to 1,000 mg/kg/day (9 times the recommended maximum human dose based on mg/m2) have revealed no impairment of fertility.
Pregnancy
Teratogenic Effects: Pregnancy Category B. Reproduction studies have been performed in mice at doses up to 3,200 mg/kg/day (14 times the recommended maximum human dose based on mg/m2) and in rats at doses up to 1,000 mg/kg/day (9 times the recommended maximum human dose based on mg/m2) and have revealed no evidence of impaired fertility or harm to the fetus due to cefuroxime axetil. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Nursing Mothers
Because cefuroxime is excreted in human milk, consideration should be given to discontinuing nursing temporarily during treatment with cefuroxime axetil.
Pediatric Use
The safety and effectiveness of cefuroxime axetil have been established for pediatric patients aged 3 months to 12 years for acute bacterial maxillary sinusitis based upon its approval in adults. Use of cefuroxime axetil in pediatric patients is supported by pharmacokinetic and safety data in adults and pediatric patients, and by clinical and microbiological data from adequate and well-controlled studies of the treatment of acute bacterial maxillary sinusitis in adults and of acute otitis media with effusion in pediatric patients. It is also supported by postmarketing adverse events surveillance (see CLINICAL PHARMACOLOGY, INDICATIONS AND USAGE, ADVERSE REACTIONS, DOSAGE AND ADMINISTRATION, and CLINICAL STUDIES).
Geriatric Use
Of the total number of subjects who received cefuroxime axetil in 20 clinical studies of cefuroxime axetil, 375 were 65 and over while 151 were 75 and over. No overall differences in safety or effectiveness were observed between these subjects and younger adult subjects. The geriatric patients reported somewhat fewer gastrointestinal events and less frequent vaginal candidiasis compared with patients aged 12 to 64 years old; however, no clinically significant differences were reported between the elderly and younger adult patients. Other reported clinical experience has not identified differences in responses between the elderly and younger adult patients.
-
ADVERSE REACTIONS
In clinical trials using multiple doses of cefuroxime axetil powder for oral suspension, pediatric patients (96.7% of whom were younger than 12 years of age) were treated with the recommended dosages of cefuroxime axetil (20 to 30 mg/kg/day divided twice a day up to a maximum dose of 500 or 1,000 mg/day, respectively). There were no deaths or permanent disabilities in any of the patients in these studies. Eleven U.S. patients (1.2%) discontinued medication due to adverse events thought by the investigators to be possibly, probably, or almost certainly related to drug toxicity. The discontinuations were primarily for gastrointestinal disturbances, usually diarrhea or vomiting. During clinical trials, discontinuation of therapy due to the taste and/or problems with administering this drug occurred in 13 (1.4%) pediatric patients enrolled at centers in the United States.
The following adverse events were thought by the investigators to be possibly, probably, or almost certainly related to cefuroxime axetil for oral suspension in multiple-dose clinical trials (n = 931 cefuroxime axetil-treated U.S. patients).
Table 3: Adverse Reactions—Cefuroxime Axetil For Oral Suspension Multiple-Dose Dosing Regimens—Clinical Trials Incidence ≥ 1% Diarrhea/loose stools 8.6% Dislike of taste 5 % Diaper rash 3.4% Nausea/vomiting 2.6% Incidence < 1% but > 0.1% Abdominal pain Flatulence Gastrointestinal infection Candidiasis Vaginal irritation Rash Hyperactivity Irritable behavior Eosinophilia Positive direct Coombs’ test Elevated liver enzymes Viral illness Upper respiratory infection Sinusitis Cough Urinary tract infection Joint swelling Arthralgia Fever Ptyalism POSTMARKETING EXPERIENCE WITH CEFUROXIME AXETIL PRODUCTS
In addition to adverse events reported during clinical trials, the following events have been identified during clinical practice in patients treated with cefuroxime tablets or with cefuroxime axetil for oral suspension and were reported spontaneously. Data are generally insufficient to allow an estimate of incidence or to establish causation.
General:The following hypersensitivity reactions have been reported: anaphylaxis, angioedema, pruritis, rash, serum sickness-like reaction, and urticaria.
Gastrointestinal: Pseudomembranous colitis (see WARNINGS).
Hematologic: Hemolytic anemia, leukopenia, pancytopenia, thrombocytopenia, and increased prothrombin time.
Hepatic: Hepatic impairment including hepatitis and cholestasis, jaundice.
Skin:Erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis.
CEPHALOSPORIN-CLASS ADVERSE REACTIONS
In addition to the adverse reactions listed above that have been observed in patients treated with cefuroxime axetil, the following adverse reactions and altered laboratory tests have been reported for cephalosporin-class antibiotics: toxic nephropathy, aplastic anemia, hemorrhage, increased BUN, increased creatinine, false-positive test for urinary glucose, increased alkaline phosphatase, neutropenia, elevated bilirubin, and agranulocytosis.
Several cephalosporins have been implicated in triggering seizures, particularly in patients with renal impairment when the dosage was not reduced (see DOSAGE AND ADMINISTRATION and OVERDOSAGE). If seizures associated with drug therapy occur, the drug should be discontinued. Anticonvulsant therapy can be given if clinically indicated.
- OVERDOSAGE
-
DOSAGE AND ADMINISTRATION
NOTE: CEFUROXIME AXETIL TABLETS AND CEFUROXIME AXETIL FOR ORAL SUSPENSION ARE NOT BIOEQUIVALENT AND ARE NOT SUBSTITUTABLE ON A MILLIGRAM-PER-MILLIGRAM BASIS (SEE CLINICAL PHARMACOLOGY).
Cefuroxime axetil for oral suspension may be administered to pediatric patients ranging in age from 3 months to 12 years, according to dosages in Table 4:
Table 4: Cefuroxime Axetil For Oral Suspension(Must be administered with food. Shake well each time before using.) Population/Infection Dosage Daily Maximum Dose Duration (days) Pediatric Patients (3 months to 12 years) Pharyngitis/tonsillitis 20 mg/kg/day divided b.i.d. 500 mg 10 Acute otitis media 30 mg/kg/day divided b.i.d. 1,000 mg 10 Acute bacterial maxillary sinusitis 30 mg/kg/day divided b.i.d. 1,000 mg 10 Impetigo 30 mg/kg/day divided b.i.d. 1,000 mg 10 Patients With Renal Failure: The safety and efficacy of cefuroxime axetil in patients with renal failure have not been established. Since cefuroxime is renally eliminated, its half-life will be prolonged in patients with renal failure.
Directions for Mixing Cefuroxime Axetil For Oral Suspension: Prepare a suspension at the time of dispensing as follows:
1. Shake the bottle to loosen the powder.
3. Add the total amount of water for reconstitution (see Table 5) and replace the cap.
4. Vigorously shake in a diagonal direction to form suspension.
Table 5: Amount of Water Required for Reconstitution of Labeled Volumes of Cefuroxime Axetil For Oral Suspension Cefuroxime Axetil for Oral Suspension Labeled Volume After Reconstitution Amount of Water Required for Reconstitution 125 mg/5 mL 50 mL 23 mL 100 mL 44 mL 250 mg/5 mL 50 mL 22 mL 100 mL 43 mL NOTE: SHAKE THE ORAL SUSPENSION WELL BEFORE EACH USE. Replace cap securely after each opening. Store the reconstituted suspension between 2 and 8°C (36 and 46°F) (in the refrigerator). DISCARD AFTER 10 DAYS.
-
HOW SUPPLIED
Cefuroxime Axetil For Oral Suspension USP: When reconstituted as directed, cefuroxime axetil for oral suspension USP provides the equivalent of 125 mg or 250 mg of cefuroxime (as cefuroxime axetil) per 5 mL of suspension. It is supplied as follows:
The 125 mg/5 mL: is a white to cream colored granular powder forming white to cream colored suspension on constitution with water. The resulting suspension has a fruity flavor.
NDC 63304-963-03 50 mL bottles
NDC 63304-963-04 100 mL bottles
The 250 mg/5 mL: is a white to cream colored granular powder forming white to cream colored suspension on constitution with water. The resulting suspension has a fruity flavor.
NDC 63304-964-03 50 mL bottles
NDC 63304-964-04 100 mL bottles
Before reconstitution, store dry powder between 20 - 25° C (68 - 77° F). (See USP Controlled Room Temperature)
After reconstitution, immediately store suspension between 2 - 8° C (36 - 46° F), in a refrigerator. DISCARD AFTER 10 DAYS.
-
CLINICAL STUDIES
Cefuroxime Axetil Tablets:Acute Bacterial Maxillary Sinusitis: One adequate and well-controlled study was performed in patients with acute bacterial maxillary sinusitis. In this study each patient had a maxillary sinus aspirate collected by sinus puncture before treatment was initiated for presumptive acute bacterial sinusitis. All patients had to have radiographic and clinical evidence of acute maxillary sinusitis. As shown in the following summary of the study, the general clinical effectiveness of cefuroxime axetil tablets was comparable to an oral antimicrobial agent that contained a specific beta-lactamase inhibitor in treating acute maxillary sinusitis. However, sufficient microbiology data were obtained to demonstrate the effectiveness of cefuroxime axetil tablets in treating acute bacterial maxillary sinusitis due only to Streptococcus pneumoniae or non-beta-lactamase-producing Haemophilus influenzae. An insufficient number of beta-lactamase- producing Haemophilus influenzae and Moraxella catarrhalis isolates were obtained in this trial to adequately evaluate the effectiveness of cefuroxime axetil tablets in the treatment of acute bacterial maxillary sinusitis due to these 2 organisms.
This study enrolled 317 adult patients, 132 patients in the United States and 185 patients in South America. Patients were randomized in a 1:1 ratio to cefuroxime axetil 250 mg twice daily or an oral antimicrobial agent that contained a specific beta-lactamase inhibitor. An intent-to-treat analysis of the submitted clinical data yielded the following results:
Table 6: Clinical Effectiveness of Cefuroxime Axetil Tablets Compared to Beta-Lactamase Inhibitor-Containing Control Drug in the Treatment of Acute Bacterial Maxillary Sinusitis U.S. Patients* South American Patients† Cefuroxime Axetil (n = 49) Control (n = 43) Cefuroxime Axetil (n = 87) Control (n = 89) Clinical success (cure + improvement) 65% 53% 77% 74% Clinical cure 53% 44% 72% 64% Clinical improvement 12% 9% 5% 10% * 95% Confidence interval around the success difference [-0.08, +0.32].
† 95% Confidence interval around the success difference [-0.10, +0.16].
In this trial and in a supporting maxillary puncture trial, 15 evaluable patients had non-beta-lactamase-producing Haemophilus influenzae as the identified pathogen. Ten (10) of these 15 patients (67%) had their pathogen (non-beta-lactamase-producing Haemophilus influenzae) eradicated. Eighteen (18) evaluable patients had Streptococcus pneumoniae as the identified pathogen. Fifteen (15) of these 18 patients (83%) had their pathogen (Streptococcus pneumoniae) eradicated.
Safety: The incidence of drug-related gastrointestinal adverse events was statistically significantly higher in the control arm (an oral antimicrobial agent that contained a specific beta-lactamase inhibitor) versus the cefuroxime axetil arm (12% versus 1%, respectively; P < .001), particularly drug-related diarrhea (8% versus 1%, respectively; P = .001).
Early Lyme Disease: Two adequate and well-controlled studies were performed in patients with early Lyme disease. In these studies all patients had to present with physician-documented erythema migrans, with or without systemic manifestations of infection. Patients were randomized in a 1:1 ratio to a 20-day course of treatment with cefuroxime axetil 500 mg twice daily or doxycycline 100 mg 3 times daily. Patients were assessed at 1 month posttreatment for success in treating early Lyme disease (Part I) and at 1 year posttreatment for success in preventing the progression to the sequelae of late Lyme disease (Part II).
A total of 355 adult patients (181 treated with cefuroxime axetil and 174 treated with doxycycline) were enrolled in the 2 studies. In order to objectively validate the clinical diagnosis of early Lyme disease in these patients, 2 approaches were used: 1) blinded expert reading of photographs, when available, of the pretreatment erythema migrans skin lesion; and 2) serologic confirmation (using enzyme-linked immunosorbent assay [ELISA] and immunoblot assay [“Western” blot]) of the presence of antibodies specific to Borrelia burgdorferi, the etiologic agent of Lyme disease. By these procedures, it was possible to confirm the physician diagnosis of early Lyme disease in 281 (79%) of the 355 study patients. The efficacy data summarized below are specific to this “validated” patient subset, while the safety data summarized below reflect the entire patient population for the 2 studies.
Analysis of the submitted clinical data for evaluable patients in the “validated” patient subset yielded the following results:
Table 7: Clinical Effectiveness of Cefuroxime Axetil Tablets Compared to Doxycycline in the Treatment of Early Lyme Disease Part I (1 Month Posttreatment)* Part II (1 Year Posttreatment)† Cefuroxime Axetil Doxycycline Cefuroxime Axetil Doxycycline (n = 125) (n = 108) (n = 105‡) (n = 83‡) Satisfactory clinical outcome§ 91% 93% 84% 87% Clinical cure/success 72% 73% 73% 73% Clinical improvement 19% 19% 10% 13% * 95% confidence interval around the satisfactory difference for Part I (-0.08, +0.05).
† 95% confidence interval around the satisfactory difference for Part II (-0.13, +0.07).
‡ n’s include patients assessed as unsatisfactory clinical outcomes (failure + recurrence) in Part I (cefuroxime axetil - 11 [5 failure, 6 recurrence]; doxycycline - 8 [6 failure, 2 recurrence]).
§ Satisfactory clinical outcome includes cure + improvement (Part I) and success + improvement (Part II).
Cefuroxime axetil and doxycycline were effective in prevention of the development of sequelae of late Lyme disease.
Safety: Drug-related adverse events affecting the skin were reported significantly more frequently by patients treated with doxycycline than by patients treated with cefuroxime axetil (12% versus 3%, respectively; P = .002), primarily reflecting the statistically significantly higher incidence of drug-related photosensitivity reactions in the doxycycline arm versus the cefuroxime axetil arm (9% versus 0%, respectively; P < .001). While the incidence of drug-related gastrointestinal adverse events was similar in the 2 treatment groups (cefuroxime axetil - 13%; doxycycline - 11%), the incidence of drug-related diarrhea was statistically significantly higher in the cefuroxime axetil arm versus the doxycycline arm (11% versus 3%, respectively; P = .005).
Secondary Bacterial Infections of Acute Bronchitis: Four randomized, controlled clinical studies were performed comparing 5 days versus 10 days of cefuroxime axetil for the treatment of patients with secondary bacterial infections of acute bronchitis. These studies enrolled a total of 1,253 patients (CAE-516 n = 360; CAE-517 n = 177; CAEA4001 n = 362; CAEA4002 n = 354). The protocols for CAE-516 and CAE-517 were identical and compared cefuroxime axetil 250 mg twice daily for 5 days, cefuroxime axetil 250 mg twice daily for 10 days, and AUGMENTIN® 500 mg 3 times daily for 10 days. These 2 studies were conducted simultaneously. CAEA4001 and CAEA4002 compared cefuroxime axetil 250 mg twice daily for 5 days, cefuroxime axetil 250 mg twice daily for 10 days, and CECLOR® 250 mg 3 times daily for 10 days. They were otherwise identical to CAE-516 and CAE-517 and were conducted over the following 2 years. Patients were required to have polymorphonuclear cells present on the Gram stain of their screening sputum specimen, but isolation of a bacterial pathogen from the sputum culture was not required for inclusion. The following table demonstrates the results of the clinical outcome analysis of the pooled studies CAE-516/CAE-517 and CAEA4001/CAEA4002, respectively:
Table 8: Clinical Effectiveness of Cefuroxime Axetil Tablets 250 mg Twice Daily in Secondary Bacterial Infections of Acute Bronchitis: Comparison of 5 Versus 10 Days’ Treatment Duration CAE-516 and CAE-517* CAEA4001 and CAEA4002† 5 Day (n = 127) 10 Day (n = 139) 5 Day (n = 173) 10 Day (n = 192) Clinical success (cure + improvement) 80% 87% 84% 82% Clinical cure 61% 70% 73% 72% Clinical improvement 19% 17% 11% 10% * 95% Confidence interval around the success difference [-0.164, +0.029].
† 95% Confidence interval around the success difference [-0.061, +0.103].
The response rates for patients who were both clinically and bacteriologically evaluable were consistent with those reported for the clinically evaluable patients.
Safety: In these clinical trials, 399 patients were treated with cefuroxime axetil for 5 days and 402 patients with cefuroxime axetil for 10 days. No difference in the occurrence of adverse events was observed between the 2 regimens.
-
REFERENCES
1. National Committee for Clinical Laboratory Standards. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically. 3rd ed. Approved Standard NCCLS Document M7-A3, Vol. 13, No. 25. Villanova, Pa: NCCLS; 1993.
2. National Committee for Clinical Laboratory Standards. Performance Standards for Antimicrobial Disk Susceptibility Tests. 4th ed. Approved Standard NCCLS Document M2-A4, Vol. 10, No. 7. Villanova, Pa: NCCLS; 1990.
CLINITEST and CLINISTIX are registered trademarks of Ames Division, Miles Laboratories, Inc.
CECLOR® is a registered trademark of Lilly.
AUGMENTIN® is a registered trademark of GlaxoSmithKline
- PACKAGE LABEL. PRINCIPAL DISPLAY PANEL.
-
INGREDIENTS AND APPEARANCE
CEFUROXIME AXETIL FOR ORAL SUSPENSION
cefuroxime axetil suspensionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:63304-963 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CEFUROXIME AXETIL (UNII: Z49QDT0J8Z) (CEFUROXIME - UNII:O1R9FJ93ED) CEFUROXIME 125 mg in 5 mL Inactive Ingredients Ingredient Name Strength ASPARTAME (UNII: Z0H242BBR1) HYPROMELLOSE PHTHALATE (24% PHTHALATE, 55 CST) (UNII: 87Y6436BKR) MANNITOL (UNII: 3OWL53L36A) METHACRYLIC ACID - METHYL METHACRYLATE COPOLYMER (1:1) (UNII: 74G4R6TH13) MONOSODIUM CITRATE (UNII: 68538UP9SE) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM CHLORIDE (UNII: 451W47IQ8X) SUCROSE (UNII: C151H8M554) XANTHAN GUM (UNII: TTV12P4NEE) Product Characteristics Color Score Shape Size Flavor TUTTI FRUTTI, PEPPERMINT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63304-963-03 50 mL in 1 BOTTLE 2 NDC:63304-963-04 100 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA065323 02/08/2008 CEFUROXIME AXETIL FOR ORAL SUSPENSION
cefuroxime axetil suspensionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:63304-964 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CEFUROXIME AXETIL (UNII: Z49QDT0J8Z) (CEFUROXIME - UNII:O1R9FJ93ED) CEFUROXIME 250 mg in 1 mL Inactive Ingredients Ingredient Name Strength ASPARTAME (UNII: Z0H242BBR1) HYPROMELLOSE PHTHALATE (24% PHTHALATE, 55 CST) (UNII: 87Y6436BKR) MANNITOL (UNII: 3OWL53L36A) METHACRYLIC ACID - METHYL METHACRYLATE COPOLYMER (1:1) (UNII: 74G4R6TH13) MONOSODIUM CITRATE (UNII: 68538UP9SE) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM CHLORIDE (UNII: 451W47IQ8X) SUCROSE (UNII: C151H8M554) XANTHAN GUM (UNII: TTV12P4NEE) Product Characteristics Color Score Shape Size Flavor TUTTI FRUTTI, PEPPERMINT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63304-964-03 50 mL in 1 BOTTLE 2 NDC:63304-964-04 100 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA065323 02/08/2008 Labeler - Ranbaxy Pharmaceuticals Inc (937890044) Registrant - Ranbaxy Pharmaceuticals Inc (937890044) Establishment Name Address ID/FEI Business Operations RANBAXY LABORATORIES LIMITED - DEWAS 862358806 manufacture(63304-963, 63304-964)