CHILDRENS IBUPROFEN- ibuprofen suspension
Chain Drug Consortium, LLC
----------
Ibuprofen Oral Suspension, USP
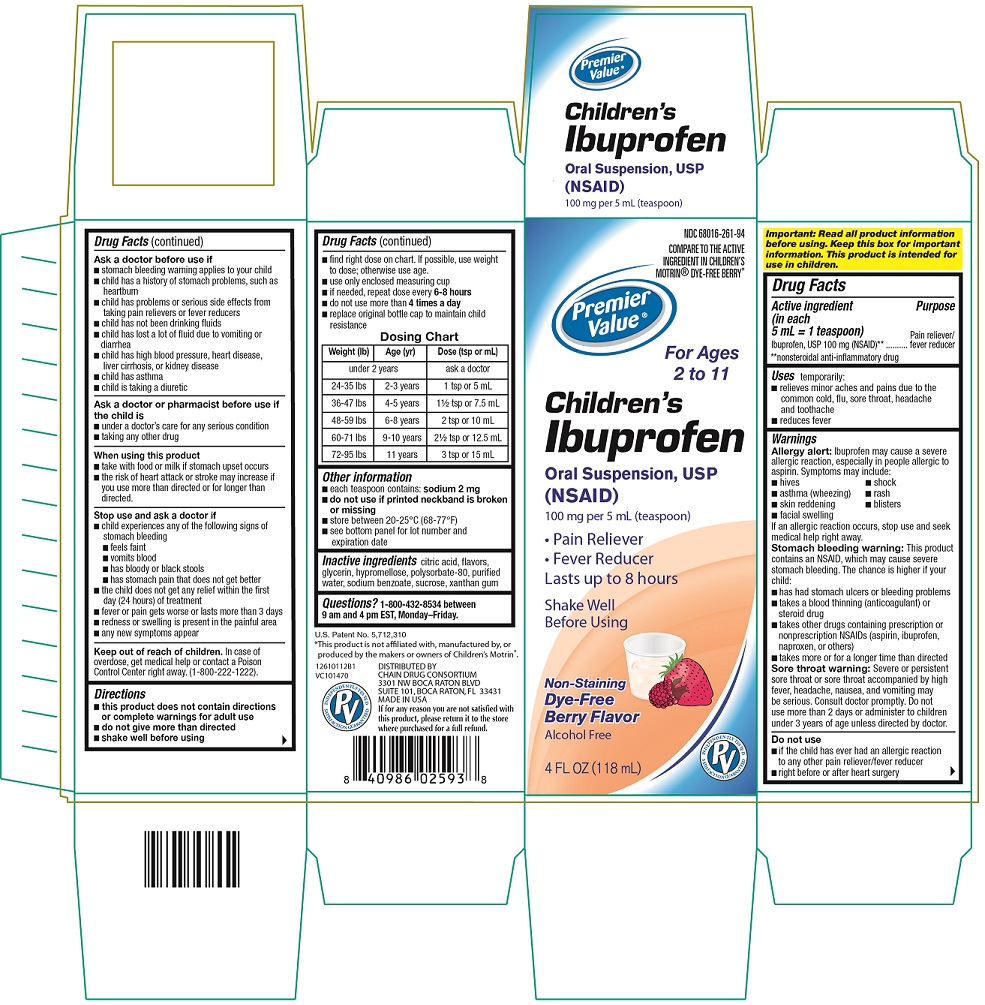
ACTIVE INGREDIENT
(in each 5 mL = 1 teaspoon)
Ibuprofen, USP 100 mg (NSAID)**
**nonsteroidal anti-inflammatory drug
USES
temporarily:
- relieves minor aches and pains due to the common cold, flu, sore throat, headache and toothache
- reduces fever
WARNINGS
Allergy alert: Ibuprofen may cause a severe allergic reaction, especially in people allergic to aspirin. Symptoms may include:
- hives
- shock
- asthma (wheezing)
- rash
- skin reddening
- blisters
- facial swelling
If an allergic reaction occurs, stop use and seek medical help right away.
Stomach bleeding warning: This product contains an NSAID, which may cause severe stomach bleeding. The chance is higher if your child:
- has had stomach ulcers or bleeding problems
- takes a blood thinning (anticoagulant) or steroid drug
- takes other drugs containing prescription or nonprescription NSAIDs (aspirin, ibuprofen, naproxen, or others)
- takes more or for a longer time than directed
Sore throat warning: Severe or persistent sore throat or sore throat accompanied by high fever, headache, nausea, and vomiting may be serious. Consult doctor promptly. Do not use more than 2 days or administer to children under 3 years of age unless directed by doctor.
DO NOT USE:
- if the child has ever had an allergic reaction to any other pain reliever/fever reducer
- right before or after heart surgery
ASK DOCTOR BEFORE USE IF:
- stomach bleeding warning applies to your child
- child has a history of stomach problems, such as heartburn
- child has problems or serious side effects from taking pain relievers or fever reducers
- child has not been drinking fluids
- child has lost a lot of fluid due to vomiting or diarrhea
- child has high blood pressure, heart disease, liver cirrhosis, or kidney disease
- child has asthma
- child is taking a diuretic
ASK DOCTOR OR PHARMACIST BEFORE USE IF THE CHILD IS:
- under a doctor’s care for any serious condition
- taking any other drug
WHEN USING THIS PRODUCT:
- take with food or milk if stomach upset occurs
- the risk of heart attack or stroke may increase if you use more than directed or for longer than directed
STOP USE AND ASK A DOCTOR IF:
- child experiences any of the following signs of stomach bleeding
- feels faint
- ·vomits blood
- has bloody or black stools
- has stomach pain that does not get better
- the child does not get any relief within the first day (24 hours) of treatment
- fever or pain gets worse or lasts more than 3 days
- redness or swelling is present in the painful area
- any new symptoms appear
KEEP OUT OF REACH OF CHILDREN
In case of overdose, get medical help or contact a Poison Control Center right away. (1-800-222-1222).
DIRECTIONS
- this product does not contain directions or complete warnings for adult use
- do not give more than directed
- shake well before using
- find right dose on chart. If possible, use weight to dose; otherwise use age.
- use only enclosed measuring cup
- if needed, repeat dose every 6-8 hours
- do not use more than 4 times a day
- replace original bottle cap to maintain child resistance
Dosing Chart
|
Weight (lb) |
Age (yr) |
Dose (tsp or mL) |
|
under 2 years |
ask a doctor |
|
|
24-35 lbs |
2-3 years |
1 tsp or 5 mL |
|
36-47 lbs |
4-5 years |
1 ½ tsp or 7.5 mL |
|
48-59 lbs |
6-8 years |
2 tsp or 10 mL |
|
60-71 lbs |
9-10 years |
2 ½ tsp or 12.5 mL |
|
72-95 lbs |
11 years |
3 tsp or 15 mL |
OTHER INFORMATION
- each teaspoon contains: sodium 2 mg
- do not use if printed neckband is broken or missing
- store between 20 - 25°C (68 - 77°F)
- see bottom panel for lot number and expiration date
INACTIVE INGREDIENTS
Berry flavor: citric acid, D&C yellow #10, FD&C red #40, flavors, glycerin, hypromellose, polysorbate 80, purified water, sodium benzoate, sucrose, xanthan gum
Dye free berry flavor: citric acid, flavors, glycerin, hypromellose, polysorbate-80, purified water, sodium benzoate, sucrose, xanthan gum
Grape flavor: citric acid, D&C red #33, FD&C blue #1, FD&C red #40, flavors, glycerin, hypromellose, polysorbate-80, purified water, sodium benzoate, sucrose, xanthan gum
Bubble gum flavor: artificial bubble gum flavor, citric acid, FD&C red #40, glycerin, hypromellose, polysorbate-80, purified water, sodium benzoate, sucrose, xanthan gum
Package Label - Original Berry Flavor
NDC 68016-255-94
COMPARE TO THE ACTIVE INGREDIENT IN CHILDREN’S MOTRIN®BERRY*
Premier Value®
For Ages 2 to 11
Children’s
Ibuprofen
Oral Suspension, USP
(NSAID)
100 mg per 5 mL (teaspoon)
- Pain Reliever
- Fever Reducer
Lasts up to 8 hours
Shake Well
Before Using
Original Berry Flavor
Alcohol Free
4 fl oz (118 mL)

Package Label - Dye-Free Berry Flavor
NDC 68016-261-94
COMPARE TO THE ACTIVE INGREDIENT IN CHILDREN’S MOTRIN® DYE FREE BERRY*
Premier Value®
For Ages 2 to 11
Children’s
Ibuprofen
Oral Suspension, USP
(NSAID)
100 mg per 5 mL (teaspoon)
- Pain Reliever
- Fever Reducer
Lasts up to 8 hours
Shake Well
Before Using
Non- staining
Dye free Berry Flavor
Alcohol Free
4 fl oz (118 mL)
Package Label - Grape Flavor
NDC 68016-262-94
COMPARE TO THE ACTIVE INGREDIENT IN CHILDREN’S MOTRIN® GRAPE*
Premier Value®
For Ages 2 to 11
Children’s
Ibuprofen
Oral Suspension, USP
(NSAID)
100 mg per 5 mL (teaspoon)
- Pain Reliever
- Fever Reducer
Lasts up to 8 hours
Shake Well
Before Using
Grape Flavor
Alcohol Free
4 fl oz (118 mL)

Package Label - Bupple Gum Flavor
NDC 68016-263-94
COMPARE TO THE ACTIVE INGREDIENT IN CHILDREN’S MOTRIN® BUBBLE GUM*
Premier Value®
For Ages 2 to 11
Children’s
Ibuprofen
Oral Suspension, USP
(NSAID)
100 mg per 5 mL (teaspoon)
- Pain Reliever
- Fever Reducer
Lasts up to 8 hours
Shake Well
Before Using
Bubble Gum Flavor
Alcohol Free
4 fl oz (118 mL)

| CHILDRENS IBUPROFEN
ibuprofen suspension |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| CHILDRENS IBUPROFEN
ibuprofen suspension |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| CHILDRENS IBUPROFEN
ibuprofen suspension |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| CHILDRENS IBUPROFEN
ibuprofen suspension |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Chain Drug Consortium, LLC (101668460) |
| Registrant - Teva Pharmaceuticals USA, Inc. (001627975) |