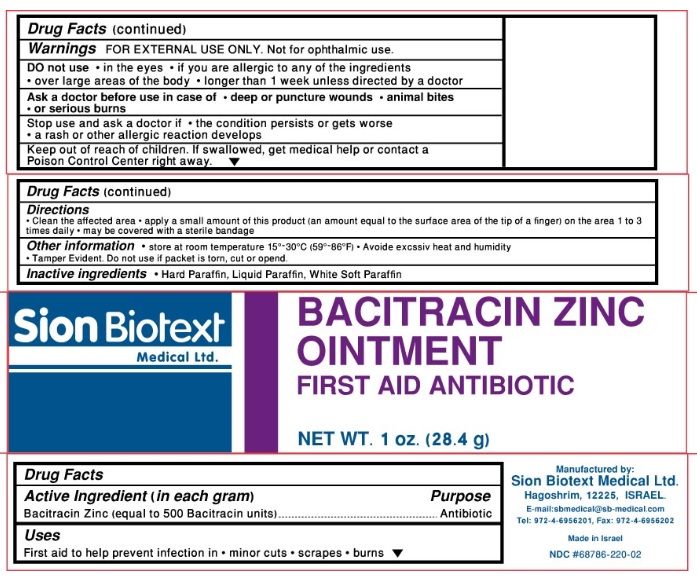
BACITRACIN ZINC- bacitracin zinc ointment
Sion Biotext Medical Ltd
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Bacitracin Zinc Ointment
Active Ingredient
Active ingredient Purpose
Bacitracin Zinc (equal to 500 Bacitracin units) Antibiotic
Other information
- store at controlled room temperature 15°-30° C (59°-86° F)
- Avoid excessive heat and humidity
- tamper evident. Do not use if packet is torn, cut or open.
Directions
- clean the affected area
- apply a small amount of this product ( an equal amount to the surface area of the tip of a finger) on the area 1 to 3 times daily
- may be covered with a sterile bandage
Stop use and ask a doctor if:
- the condition persists or gets worse
- a rash or other allergic reaction develops
| BACITRACIN ZINC
bacitracin zinc ointment |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Sion Biotext Medical Ltd (532775194) |
| Registrant - Sion Biotext Medical Ltd (532775194) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Sion Biotext Medical Ltd | 532775194 | manufacture(68786-220) | |
Revised: 12/2022
Document Id: efe17a29-c448-d452-e053-2995a90ae9bd
Set id: 120f363b-c9d1-467d-b8d4-82c2023800eb
Version: 4
Effective Time: 20221215
Sion Biotext Medical Ltd