Label: MOTION SICKNESS RELIEF- meclizine hcl tablet
- NDC Code(s): 41163-304-02
- Packager: United Natural Foods, Inc. dba UNFI
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 16, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you have
- glaucoma
- difficulty in urination due to enlargement of the prostate gland
- a breathing problem such as emphysema or chronic bronchitis
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
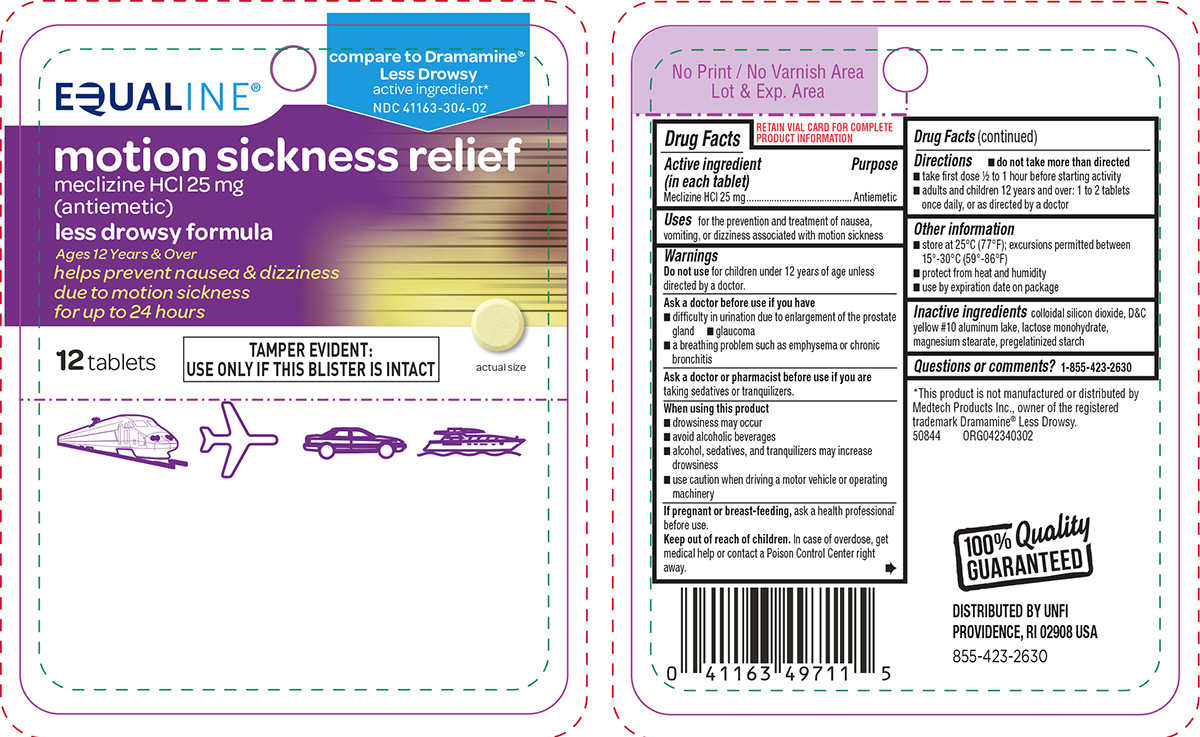
Principal Display Panel
EQUALINE®
compare to Dramamine®
Less Drowsy
active ingredient*
NDC 41163-304-02motion sickness relief
meclizine HCl 25 mg
(antiemetic)less drowsy formula
Ages 12 Years & Over
helps prevent nausea & dizziness
due to motion sickness
for up to 24 hours12 tablets
TAMPER EVIDENT:
USE ONLY IF THIS BLISTER IS INTACTactual size
*This product is not manufactured or distributed by
Medtech Products Inc., owner of the registered
trademark Dramamine® Less Drowsy.
50844 ORG042340302100% Quality
GUARANTEEDDISTRIBUTED BY UNFI
PROVIDENCE, RI 02908 USA
855-423-2630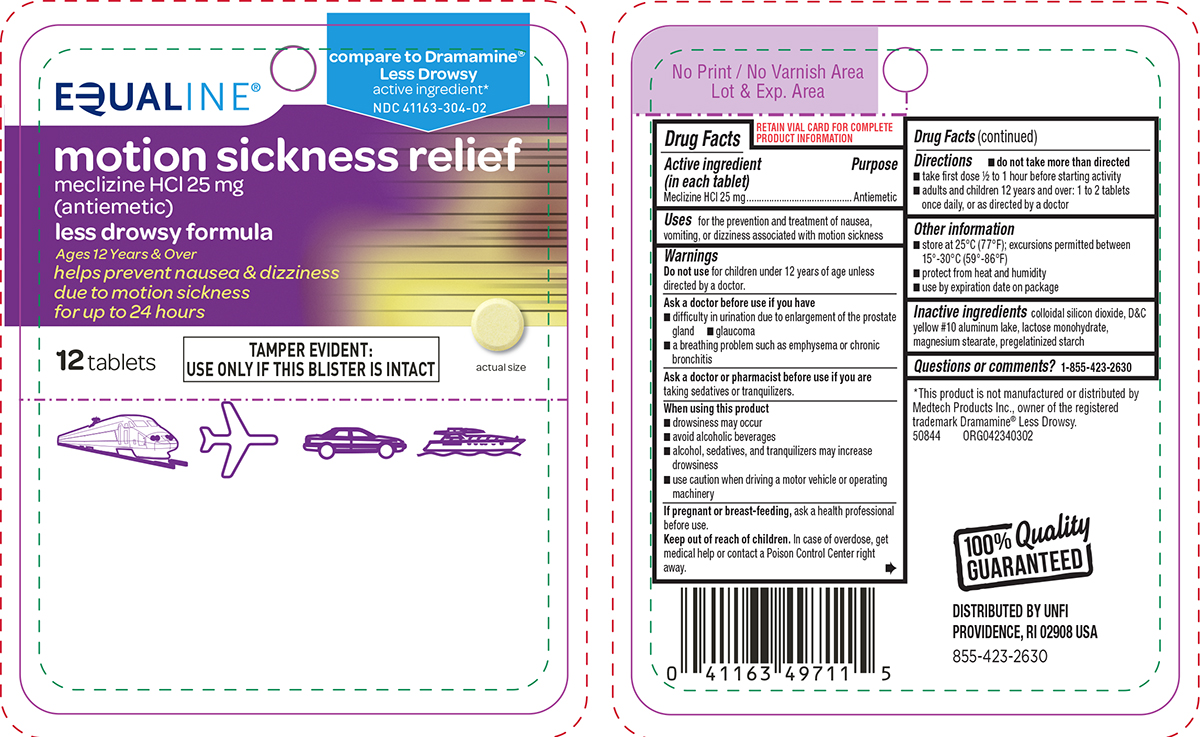
Equaline 44-403
-
INGREDIENTS AND APPEARANCE
MOTION SICKNESS RELIEF
meclizine hcl tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:41163-304 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MECLIZINE HYDROCHLORIDE (UNII: HDP7W44CIO) (MECLIZINE - UNII:3L5TQ84570) MECLIZINE HYDROCHLORIDE 25 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) D&C YELLOW NO. 10 ALUMINUM LAKE (UNII: CQ3XH3DET6) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) STARCH, CORN (UNII: O8232NY3SJ) Product Characteristics Color yellow Score no score Shape ROUND Size 9mm Flavor Imprint Code 44;403 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:41163-304-02 1 in 1 PACKAGE 06/24/2002 1 12 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M009 06/24/2002 Labeler - United Natural Foods, Inc. dba UNFI (943556183) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 038154464 pack(41163-304) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867837 manufacture(41163-304) , pack(41163-304) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 967626305 pack(41163-304) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 117025878 manufacture(41163-304)