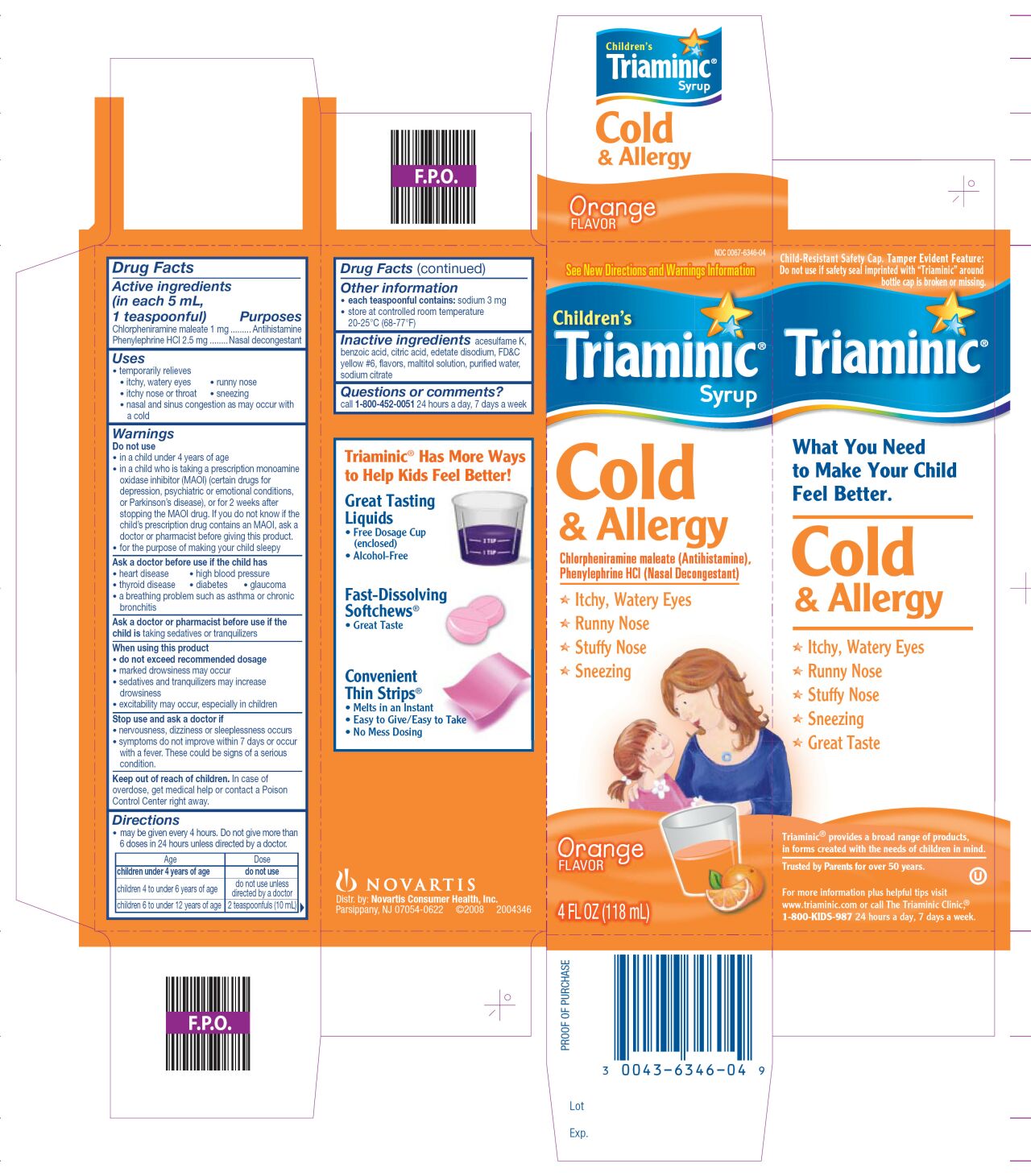
TRIAMINIC CHILDRENS COLD AND ALLERGY- chlorpheniramine maleate, phenylephrine hcl syrup
GlaxoSmithKline Consumer Healthcare Holdings (US) LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Drug Facts
Uses
- •
- temporarily relieves
- • itchy, watery eyes • runny nose • itchy nose or throat
- • sneezing • nasal and sinus congestion as may occur with a cold
Warnings
Do not use
- •
- in a child under 4 years of age
- •
- in a child who is taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if the child’s prescription drug contains an MAOI, ask a doctor or pharmacist before giving this product.
for the purpose of making your child sleepy
Ask a doctor before use if the child has
• heart disease • high blood pressure • thyroid disease
• diabetes • glaucoma
• a breathing problem such as asthma or chronic bronchitis
When using this product
- •
- do not exceed recommended dosage
- •
- marked drowsiness may occur
- •
- sedatives and tranquilizers may increase drowsiness
excitability may occur, especially in children
Directions
- •
- may be given every 4 hours. Do not give more than 6 doses in 24 hours unless directed by a doctor.
|
Age |
Dose |
|
Children under 4 years of age |
do not use |
|
children 4 to under 6 years of age |
do not use unless directed by a doctor |
|
children 6 to under 12 years of age |
2 teaspoonfuls (10 mL) |
Other information
- •
- each teaspoonful contains: sodium 3 mg
- •
- store at controlled room temperature 20-25°C (68-77°F)
Inactive ingredients
acesulfame K, benzoic acid, citric acid, edetate disodium, FD&C yellow #6, flavors, maltitol solution, purified water, sodium citrate
Additional information
Child-Resistant Safety Cap. Tamper Evident Feature: Do not use if safety seal imprinted with “Triaminic” around the bottle cap is broken or missing.
Triaminic® provides a broad range of products, in forms created with the needs of children in mind.
Trusted by Parents for over 50 years. (Depending on space availability)
For more information plus helpful tips visit www.triaminic.com or call The Triaminic Clinic®
1-800-KIDS-987 24 hours a day, 7 days a week. (Depending on space availability)
Distr. by: Novartis Consumer Health, Inc.
Parsippany, NJ 07054-0622 ©2008 2004346
| TRIAMINIC
CHILDRENS COLD AND ALLERGY
chlorpheniramine maleate, phenylephrine hcl syrup |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - GlaxoSmithKline Consumer Healthcare Holdings (US) LLC (079944263) |