CARMEX ORIGINAL LIP BALM EXTERNAL ANALGESIC
- camphor (natural), menthol salve
Carma Laboratories, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
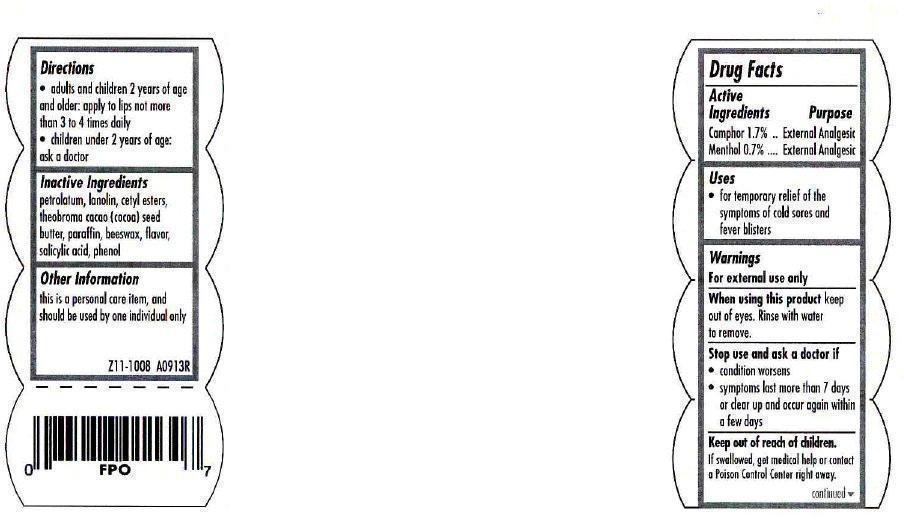
CARMEX original lip balm EXTERNAL ANALGESIC
Warnings
For external use only. When using this product, keep out of eyes. Rinse with water to remove. Stop use and consult a doctor if: condition worsens or rash occurs.
Directions
- apply to lips not more than 3 to 4 times daily
- children under 2 years of age: ask a doctor
| CARMEX ORIGINAL LIP BALM EXTERNAL ANALGESIC
camphor (natural), menthol salve |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Carma Laboratories, Inc. (006090153) |
| Registrant - Carma Laboratories, Inc. (006090153) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Carma Laboratories, Inc. | 006090153 | manufacture(10210-0011) | |
Revised: 11/2016
Document Id: 41fcfe99-460c-1524-e054-00144ff8d46c
Set id: 0f5b2c4c-55f2-4140-b706-f260e2c96cbc
Version: 4
Effective Time: 20161123
Carma Laboratories, Inc.