POVIDONE-IODINE ANTISEPTIC- povidone-iodine solution
POVIDONE-IODINE- povidone-iodine solution
Medline Industries, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
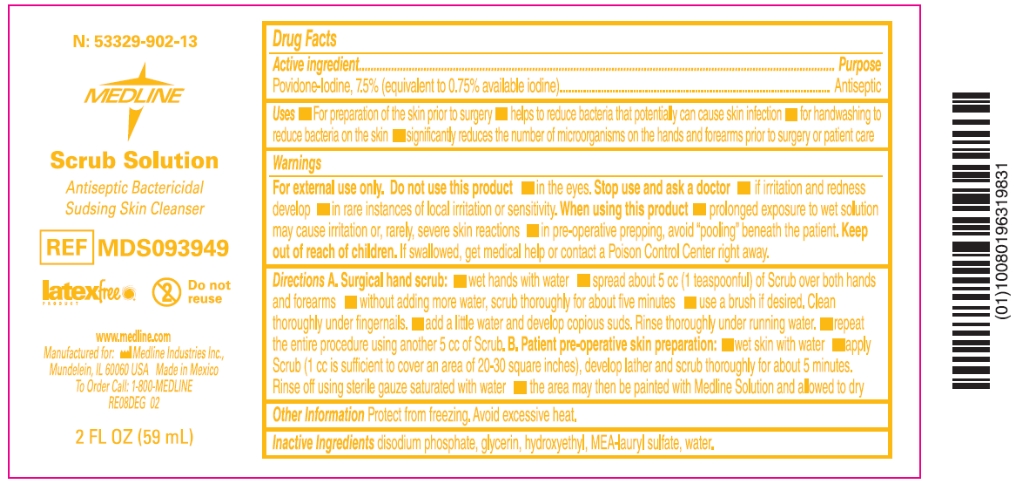
Drug Facts
Uses
- for preparation of the skin prior to surgery
- helps to reduce bacteria that potentially can cause skin infection
- for handwashing to reduce bacteria on the skin
- significantly reduces the number of microorganisms on the hands and forearms prior to surgery or patient care
Warnings
For external use only
When using this product
- prolonged exposure to wet solution may cause irritation or, rarely, severe skin reactions
- in pre-operative prepping, avoid “pooling” beneath the patient
Directions
Surgical hand scrub:
- Clean under nails with a nail pick. Nails should be maintained with a 1 millimeter free edge.
- Wet hands and forearms
- Apply 5 milliliters (teaspoonful) or palmful to hands and forearms
- Scrub thoroughly for about 5 minutes paying particular attention to the nails, cuticles, and interdigital spaces
- Use a sterile scrub brush, if desired
- Rinse and repeat scrub
Patient Pre-operative Skin Preparation:
- Prior to surgery, wet skin with water
- Apply scrub to the operative site
- Scrub thoroughly for about 5 minutes
- Rinse using sterile gauze or towel, or remove with 70 percent alcohol
- Follow with application of Medline Prep solution and allow to dry
Inactive ingredients
Citric Acid, Cocamide DEA, Disodium Phosphate, Sodium Hydroxide, Sodium Lauryl Sulfate, Water
| POVIDONE-IODINE
ANTISEPTIC
povidone-iodine solution |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| POVIDONE-IODINE
povidone-iodine solution |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Medline Industries, Inc. (025460908) |
Revised: 2/2016
Document Id: 2b947c81-92a2-28c2-e054-00144ff8d46c
Set id: 0f5a2182-9559-4ddd-8dc4-4c8e34796a54
Version: 5
Effective Time: 20160212
Medline Industries, Inc.