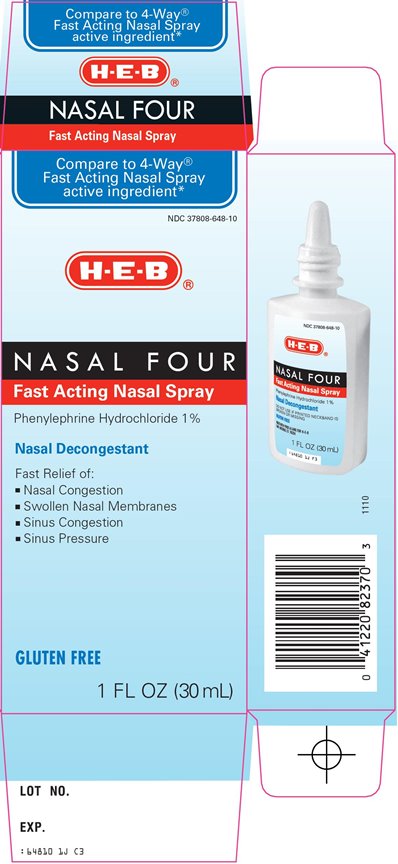
RX ACT NASAL FOUR- phenylephrine hydrochloride spray
H E B
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
HEB Nasal Four Drug Facts
Uses
- •
- temporarily relieves nasal congestion due to:
- •
- common cold
- •
- hay fever
- •
- upper respiratory allergies
Warnings
Ask a doctor before use if you have
- •
- heart disease
- •
- diabetes
- •
- thyroid disease
- •
- high blood pressure
- •
- trouble urinating due to an enlarged prostate gland
When using this product
- •
- do not use more than directed
- •
- do not use for more than 3 days. Frequent or prolonged use may cause nasal congestion to come back or get worse.
- •
- use only as directed
- •
- temporary discomfort such as burning, stinging, sneezing or an increase in nasal discharge may occur
- •
- use of this container by more than one person may spread infection
Directions
- •
- adults and children 12 years and over: 2 or 3 sprays in each nostril not more often than every 4 hours
- •
- children under 12 years: ask a doctor
Use instructions: with head in a normal, upright position, put atomizer tip into nostril. Squeeze bottle with firm, quick pressure while inhaling. Wipe nozzle clean after each use.
| RX ACT NASAL FOUR
phenylephrine hydrochloride spray |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - H E B (007924756) |
Revised: 11/2017
Document Id: 21046b04-74ea-4910-8809-e752accba2f4
Set id: 0ed34ada-5d58-4cd8-8763-d552c74a7a1e
Version: 3
Effective Time: 20171120
H E B