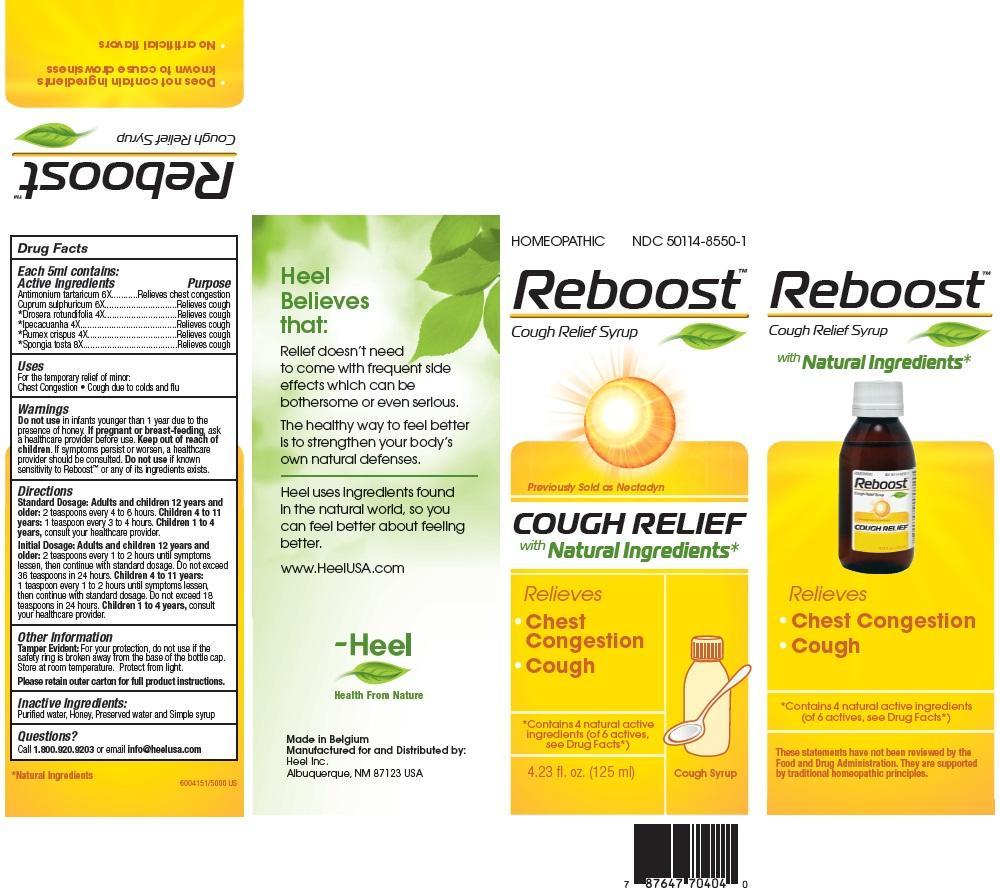
Label: REBOOST- antimony potassium tartrate syrup
-
Contains inactivated NDC Code(s)
NDC Code(s): 50114-8550-1 - Packager: Heel Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated February 13, 2013
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENTS
- INACTIVE INGREDIENTS
-
PURPOSE
Antimonium tartaricum 6X..............Relieves chest congestion
Cuprum sulphuricum 6X.................Relieves cough
Drosera rotundifolia 4X..................Relieves cough
Ipecacuanha 4X.............................Relieves cough
Rumex crispus 4X...........................Relieves cough
Spongia tosta 8X...........................Relieves cough
-
DOSAGE AND ADMINISTRATION
Standard Dosage: Adults and children 12 years and older: 2 teaspoons every 4 to 6 hours.
Children 4 to 11 tears: 1 teaspoon every 3 to 4 hours.
Children 1 to 4 years, consult your healthcare provider.
Initial Dosage: Adults and children 12 years and older: 2 teaspoons every 1 to 2 hours until symptoms lessen, then continue with standard dosage. Do not exceed 36 teaspoons in 24 hours.
Children 4 to 11 tears: 1 teaspoon every 1 to 2 hours until symptoms lessen, then continue with standard dosage. Do not exceed 18 teaspoons in 24 hours.
Children 1 to 4 years, consult your healthcare provider.
-
WARNINGS
Do not use in infants younger than 1 year due to the presence of honey. If pregnant or breast-feeding, ask a healthcare provider before use. Keep out of reach of children. If symptoms persist or worsen, a healthcare provider should be consulted. Do not use if known sensitivity to Reboost or any of its ingredients exists.
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS AND USAGE
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
REBOOST
antimony potassium tartrate syrupProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50114-8550 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ANTIMONY POTASSIUM TARTRATE (UNII: DL6OZ476V3) (ANTIMONY CATION (3+) - UNII:069647RPT5) ANTIMONY POTASSIUM TARTRATE 6 [hp_X] in 125 mL CUPRIC SULFATE (UNII: LRX7AJ16DT) (CUPRIC CATION - UNII:8CBV67279L) CUPRIC CATION 6 [hp_X] in 125 mL DROSERA ROTUNDIFOLIA (UNII: QR44N9XPJQ) (DROSERA ROTUNDIFOLIA - UNII:QR44N9XPJQ) DROSERA ROTUNDIFOLIA 4 [hp_X] in 125 mL IPECAC (UNII: 62I3C8233L) (IPECAC - UNII:62I3C8233L) IPECAC 4 [hp_X] in 125 mL RUMEX CRISPUS ROOT (UNII: 9N1RM2S62C) (RUMEX CRISPUS ROOT - UNII:9N1RM2S62C) RUMEX CRISPUS ROOT 4 [hp_X] in 125 mL SPONGIA OFFICINALIS SKELETON, ROASTED (UNII: 1PIP394IID) (SPONGIA OFFICINALIS SKELETON, ROASTED - UNII:1PIP394IID) SPONGIA OFFICINALIS SKELETON, ROASTED 8 [hp_X] in 125 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) HONEY (UNII: Y9H1V576FH) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) SUCROSE (UNII: C151H8M554) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50114-8550-1 1 in 1 CARTON 1 125 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 02/13/2013 Labeler - Heel Inc (102783016) Establishment Name Address ID/FEI Business Operations Heel Belgium 282761204 manufacture(50114-8550)