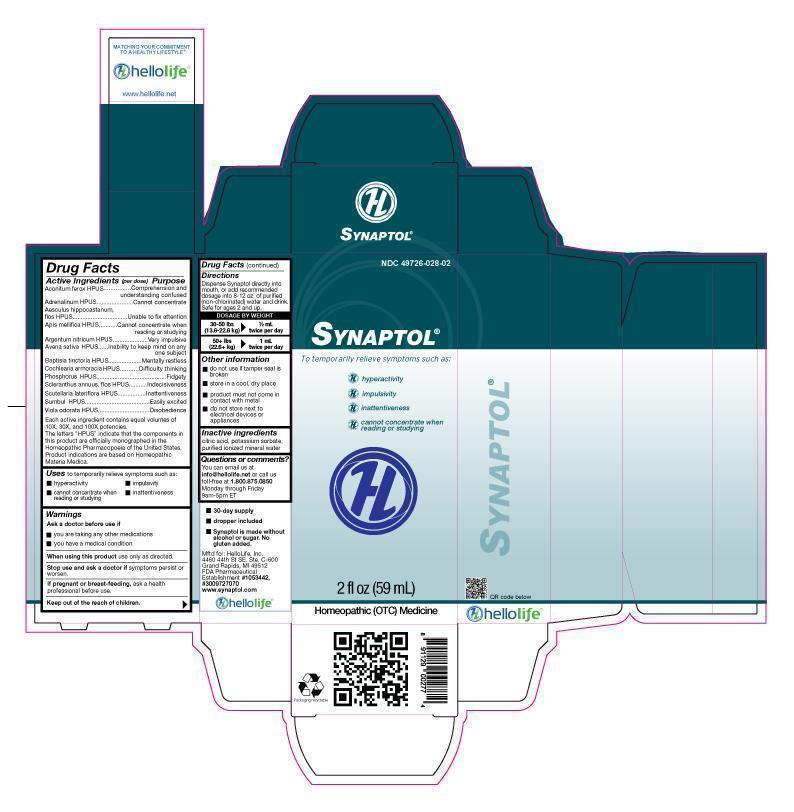
Label: SYNAPTOL- aconitum ferox, adrenallnum, aesculus hippocastanum, apis mellifica, argentum nitricum, avena sativa, baptisia tinctoria, cochlearia armoracia, phosphorus, scleranthus annuus, scutellaria lateriflora, sumbul, viola odorata liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 49726-028-02 - Packager: Hello Life, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated December 26, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Active Ingredients (per dose)
Aconitum ferox HPUS
Adrenalinum HPUS
Aesculus hippocastanum flos HPUS
Apis mellifica HPUS
Argentum nitricum HPUS
Avena sativa HPUS
Baptisia tinctoria HPUS
Cochlearia armoracia HPUS
Phosphorus HPUS
Scleranthus annuus, flos HPUS
Scutellaria lateriflora HPUS
Sumbul HPUS
Viola odorata HPUS
Each active ingredient contains equal volumes of 10X, 30X, and 100X potencies. The letters "HPUS" indicate that the components in this product are officially monographed in the Homeopathic Pharmacopoeia of the United States. Product indications are based on Homeopathic Materia Medica.
-
Purpose
Aconitum ferox HPUS........................................Comprehension and understanding confused
Adrenalinum HPUS...................................Cannot concentrate
Aesculus hippocastanum flos HPUS......................Unable to fix attention
Apis mellifica HPUS...........................Cannot concentrate when reading or studying
Argentum nitricum HPUS.........................................Very impulsive
Avena sativa HPUS.............................................Inability to keep mind on any one subject
Baptisia tinctoria HPUS......................................Mentally resless
Cochlearia armoracia HPUS................................Difficulty thinking
Phosphorus HPUS.......................................................Fidgety
Scleranthus annuus, flos HPUS.............................Indecisiveness
Scutellaria lateriflora HPUS...................................Inattentiveness
Sumbul HPUS..............................................................Easily excited
Viola odorata HPUS.......................Disobedience
- Uses
- Warnings
- Directions
- Other information
- Inactive ingredients
- Questions or comments
- DESCRIPTION
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
SYNAPTOL
aconitum ferox, adrenallnum, aesculus hippocastanum, apis mellifica, argentum nitricum, avena sativa, baptisia tinctoria, cochlearia armoracia, phosphorus, scleranthus annuus, scutellaria lateriflora, sumbul, viola odorata liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49726-028 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACONITUM FEROX ROOT (UNII: I4G31A93ZR) (ACONITUM FEROX ROOT - UNII:I4G31A93ZR) ACONITUM FEROX ROOT 100 [hp_X] in 59 mL EPINEPHRINE (UNII: YKH834O4BH) (EPINEPHRINE - UNII:YKH834O4BH) EPINEPHRINE 100 [hp_X] in 59 mL AESCULUS HIPPOCASTANUM FLOWER (UNII: KK0Z92II8M) (AESCULUS HIPPOCASTANUM FLOWER - UNII:KK0Z92II8M) AESCULUS HIPPOCASTANUM FLOWER 100 [hp_X] in 59 mL APIS MELLIFERA (UNII: 7S82P3R43Z) (APIS MELLIFERA - UNII:7S82P3R43Z) APIS MELLIFERA 100 [hp_X] in 59 mL SILVER NITRATE (UNII: 95IT3W8JZE) (SILVER CATION - UNII:57N7B0K90A) SILVER NITRATE 100 [hp_X] in 59 mL AVENA SATIVA FLOWERING TOP (UNII: MA9CQJ3F7F) (AVENA SATIVA FLOWERING TOP - UNII:MA9CQJ3F7F) AVENA SATIVA FLOWERING TOP 100 [hp_X] in 59 mL BAPTISIA TINCTORIA ROOT (UNII: 5EF0HWI5WU) (BAPTISIA TINCTORIA ROOT - UNII:5EF0HWI5WU) BAPTISIA TINCTORIA ROOT 100 [hp_X] in 59 mL HORSERADISH (UNII: 8DS6G120HJ) (HORSERADISH - UNII:8DS6G120HJ) HORSERADISH 100 [hp_X] in 59 mL PHOSPHORUS (UNII: 27YLU75U4W) (PHOSPHORUS - UNII:27YLU75U4W) PHOSPHORUS 100 [hp_X] in 59 mL SCLERANTHUS ANNUUS FLOWERING TOP (UNII: CC4B5WU2XX) (SCLERANTHUS ANNUUS FLOWERING TOP - UNII:CC4B5WU2XX) SCLERANTHUS ANNUUS FLOWERING TOP 100 [hp_X] in 59 mL SCUTELLARIA LATERIFLORA (UNII: 7BP4DH5PDC) (SCUTELLARIA LATERIFLORA - UNII:7BP4DH5PDC) SCUTELLARIA LATERIFLORA 100 [hp_X] in 59 mL FERULA SUMBUL ROOT (UNII: GLA4808EHQ) (FERULA SUMBUL ROOT - UNII:GLA4808EHQ) FERULA SUMBUL ROOT 100 [hp_X] in 59 mL VIOLA ODORATA (UNII: AET12U8B74) (VIOLA ODORATA - UNII:AET12U8B74) VIOLA ODORATA 100 [hp_X] in 59 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49726-028-02 1 in 1 CARTON 08/31/2012 1 59 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 08/31/2012 Labeler - Hello Life, Inc. (065619378) Establishment Name Address ID/FEI Business Operations Hello Life, Inc. 065619378 relabel(49726-028) , repack(49726-028) Establishment Name Address ID/FEI Business Operations KingBio, Inc. 617901350 manufacture(49726-028)