MOTION SICKNESS ORIGINAL FORMULA- dimenhydrinate tablet
Good Sense (Geiss, Destin & Dunn, Inc.)
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
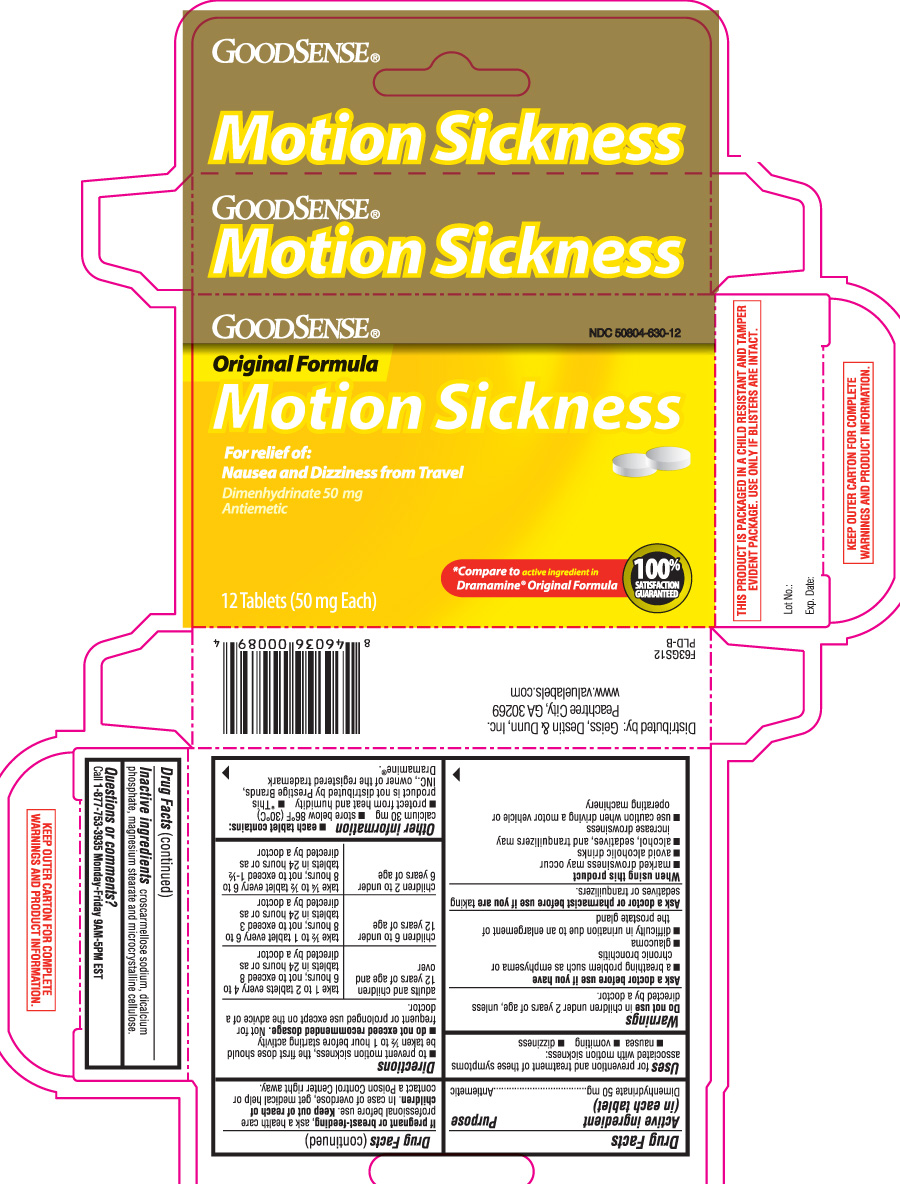
Drug Facts
Uses
for prevention and treatment of these symptoms associated with motion sickness:
- nausea
- vomiting
- dizziness
Warnings
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- difficulty in urination due to an enlargement of the prostate gland
Directions
- to prevent motion sickness, the first dose should be taken ½ to 1 hour before starting activity
- do not exceed recommended dosage. Not for frequent or prolonged use except on the advice of a doctor.
| adults and children 12 years of age and over | take 1 to 2 tablets every 4 to 6 hours; not to exceed 8 tablets in 24 hours or as directed by a doctor |
| children 6 to under 12 years of age | take ½ to 1 tablet every 6 to 8 hours; not to exceed 3 tablets in 24 hours or as directed by a doctor |
| children 2 to under 6 years of age | take ¼ to ½ tablet every 6 to 8 hours; not to exceed 1½ tablets in 24 hours or as directed by a doctor. |
Other information
- each tablet contains: calcium 30 mg
- store below 86ºF (30ºC)
- protect from heat and humidity
- *This product is not distributed by Prestige Brands, Inc., owner of the registered trademark Dramamine®.
Inactive ingredients
croscarmellose sodium, dicalcium phosphate, magnesium stearate and microcrystalline cellulose
Principal Display Panel
Original Formula
Motion Sickness
For relief of: Nausea and Dizziness from Travel
Dimenhydrinate 50 mg
Antiemetic
*Compare to active ingredient in Dramamine® Original Formula
Tablets (50 mg Each)
Distributed by: Geiss, Destin & Dunn, Inc.
Peachtree City, GA 30269
THIS PRODUCT IS PACKAGED IN A CHILD RESISTANT AND TAMPER EVIDENT PACKAGE. USE ONLY IF BLISTERS ARE INTACT.
KEEP OUTER CARTON FOR COMPLETE WARNINGS AND PRODUCT INFORMATION.
| MOTION SICKNESS
ORIGINAL FORMULA
dimenhydrinate tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Good Sense (Geiss, Destin & Dunn, Inc.) (076059836) |
| Registrant - P & L Development, LLC (079765031) |