CREST PRO-HEALTH ADVANCED WITH EXTRA TARTAR PROTECTION- sodium fluoride rinse
The Procter & Gamble Manufacturing Company
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Crest
® Pro-Health™ Advanced
with Extra Tartar Protection
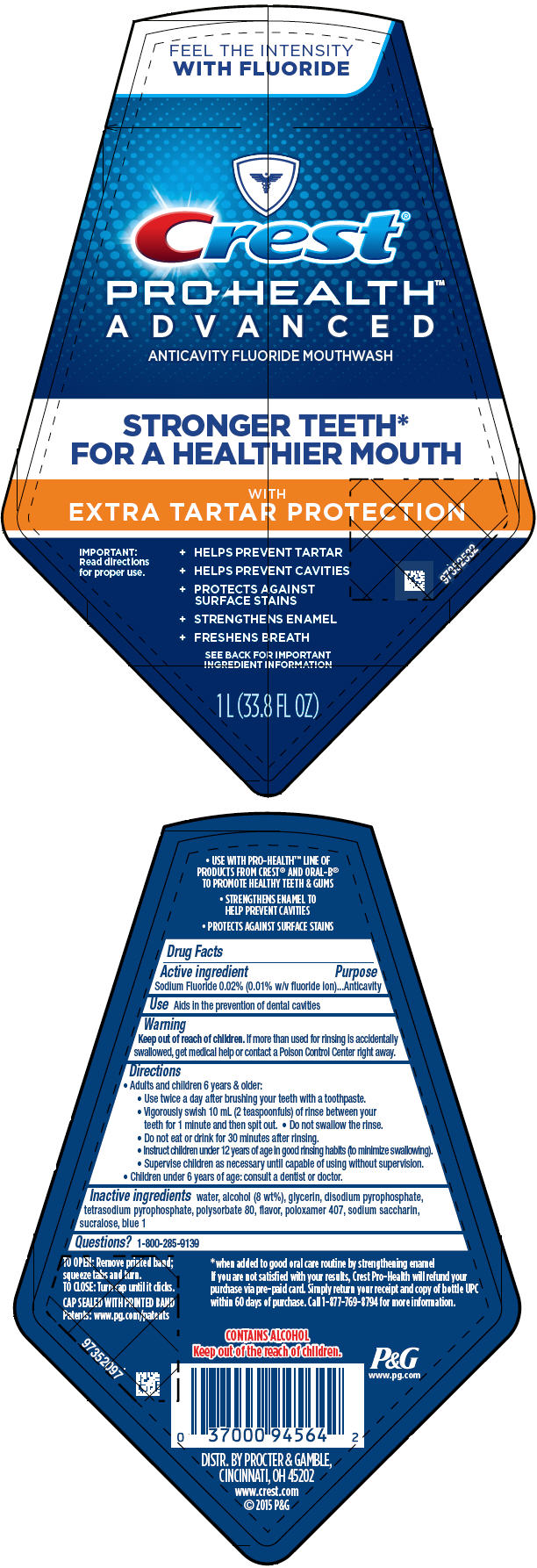
Directions
- Adults and children 6 years & older:
- Use twice a day after brushing your teeth with a toothpaste.
- Vigorously swish 10 mL (2 teaspoonfuls) of rinse between your teeth for 1 minute and then spit out.
- Do not swallow the rinse.
- Do not eat or drink for 30 minutes after rinsing.
- Instruct children under 12 years of age in good rinsing habits (to minimize swallowing).
- Supervise children as necessary until capable of using without supervision.
- Children under 6 years of age: consult a dentist or doctor.
Inactive ingredients
water, alcohol (8 wt%), glycerin, disodium pyrophosphate, tetrasodium pyrophosphate, polysorbate 80, flavor, poloxamer 407, sodium saccharin, sucralose, blue 1
PRINCIPAL DISPLAY PANEL - 1 L Bottle Label
FEEL THE INTENSITY
WITH FLUORIDE
Crest ®
PRO-HEALTH™
ADVANCED
ANTICAVITY FLUORIDE MOUTHWASH
STRONGER TEETH*
FOR A HEALTHIER MOUTH
WITH
EXTRA TARTAR PROTECTION
IMPORTANT:
Read directions
for proper use.
- HELPS PREVENT TARTAR
- HELPS PREVENT CAVITIES
- PROTECTS AGAINST
SURFACE STAINS - STRENGTHENS ENAMEL
- FRESHENS BREATH
SEE BACK FOR IMPORTANT
INGREDIENT INFORMATION
1 L (33.8 FL OZ)
97352532
| CREST PRO-HEALTH ADVANCED
WITH EXTRA TARTAR PROTECTION
sodium fluoride rinse |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - The Procter & Gamble Manufacturing Company (004238200) |