ALLERGY RELIEF ANTIHISTAMINE- diphenhydramine hcl capsule, liquid filled
TOP CARE (Topco Associates LLC)
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
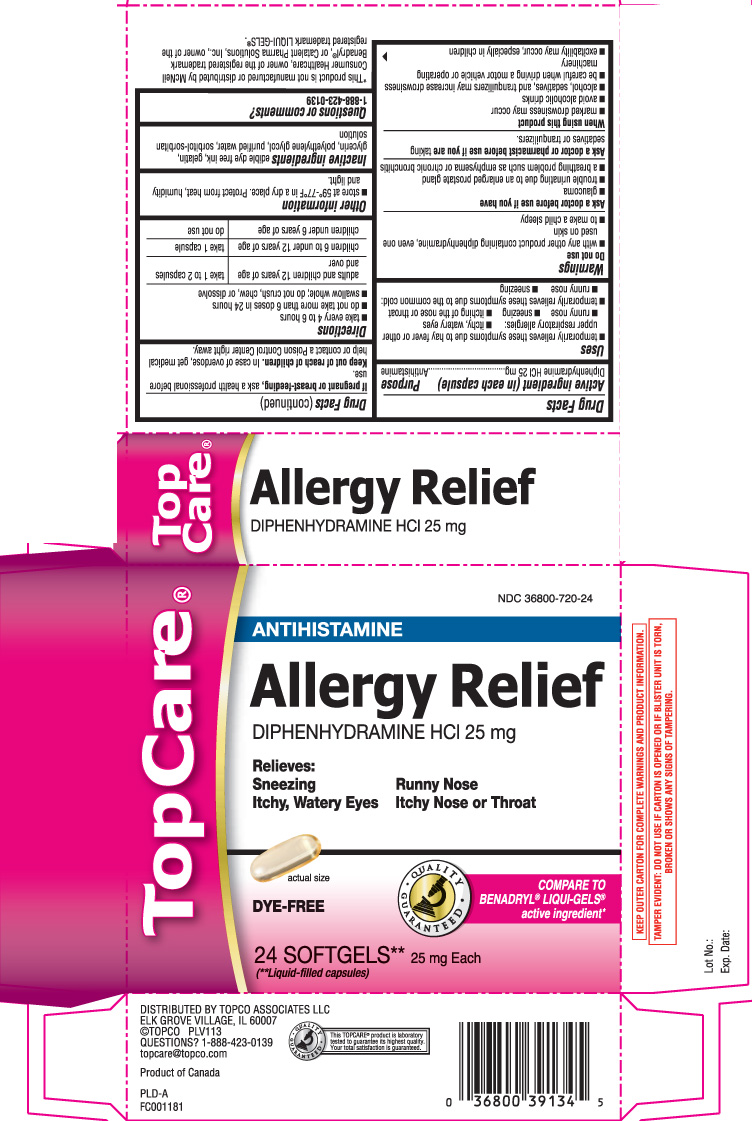
Drug Facts
Uses
- temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- itchy, watery eyes
- runny nose
- sneezing
- itching of the nose or throat
- temporarily relieves these symptoms due to the common cold:
- runny nose
- sneezing
Warnings
Do not use
- with any other product containing diphenhydramine, even one used on skin
- to make a child sleepy
Ask a doctor before use if you have
- glaucoma
- trouble urinating due to an enlarged prostate gland
- a breathing problem such as emphysema or chronic bronchitis
Directions
- take every 4 to 6 hours
- do not take more than 6 doses in 24 hours
- swallow whole; do not crush, chew or dissolve
| adults and children 12 years of age and over | take 1 to 2 capsules |
| children 6 to under 12 years of age | take 1 capsule |
| children under 6 years of age | do not use |
Inactive ingredients
edible dye free ink, gelatin, glycerin, polyethylene glycol, purified water, sorbitol-sorbitan solution
Principal Display Panel
ANTIHISTAMINE
Allergy Relief
DIPHENHYDRAMINE HCl 25 mg
Relieves:
Sneezing Runny Nose
Itchy, Watery Eyes Itchy Nose or Throat
COMPARE TO BENADRYL® LIQUI-GELS® active ingredient*
DYE-FREE
SOFTGELS** 25 mg Each
(**Liquid-filled capsules)
*This product is not manufactured or distributed by McNeil Consumer Healthcare, owner of the registered trademark Benadryl®, or Catalent Pharma Solutions, Inc., owner of the registered trademark LIQUI-GELS®.
KEEP OUTER CARTON FOR COMPLETE WARNINGS AND PRODUCT INFORMATION.
TAMPER EVIDENT: DO NOT USE IF CARTON IS OPENED OR IF BLISTER UNIT IS TORN, BROKEN OR SHOWS ANY SIGNS OF TAMPERING.
DISTRIBUTED BY TOPCO ASSOCIATES LLC
ELK GROVE VILLAGE, IL 60007
Product of Canada
| ALLERGY RELIEF
ANTIHISTAMINE
diphenhydramine hcl capsule, liquid filled |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - TOP CARE (Topco Associates LLC) (006935977) |