PRISMASOL BGK0/2.5- calcium chloride, magnesium chloride, dextrose monohydrate, lactic acid, sodium chloride, and sodium bicarbonate solution
PRISMASOL BGK4/2.5- calcium chloride, magnesium chloride, dextrose monohydrate, lactic acid, sodium chloride, sodium bicarbonate, and potassium chloride solution
PRISMASOL BGK2/3.5- calcium chloride, magnesium chloride, dextrose monohydrate, lactic acid, sodium chloride, sodium bicarbonate, and potassium chloride solution
PRISMASOL BGK2/0- magnesium chloride, dextrose monohydrate, lactic acid, sodium chloride, sodium bicarbonate, and potassium chloride solution
PRISMASOL B22GK4/0- magnesium chloride, dextrose monohydrate, lactic acid, sodium chloride, sodium bicarbonate, and potassium chloride solution
PRISMASOL BK0/0/1.2- magnesium chloride, lactic acid, sodium chloride, and sodium bicarbonate solution
PRISMASOL BGK4/0/1.2- magnesium chloride, dextrose monohydrate, lactic acid, sodium chloride, sodium bicarbonate, and potassium chloride solution
Gambro Renal Products
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use PrismaSol solution safely and effectively. See full prescribing information for PrismaSol solution.
PrismaSol® solution, for intravenous administration. Initial U.S. Approval: 2006 INDICATIONS AND USAGEPrismaSol solution is indicated: (1)
DOSAGE AND ADMINISTRATIONThe mode of therapy, solute formulations, flow rates and length of therapy should be selected by the physician responsible for managing treatment depending on the clinical condition of the patient as well as the patient's fluid, electrolyte, acid base and glucose balance. (2) DOSAGE FORMS AND STRENGTHS1000 mL of the reconstituted PrismaSol solution contains (in mEq/L except where noted): (3)
CONTRAINDICATIONSNone (4) WARNINGS AND PRECAUTIONS
ADVERSE REACTIONSRevised: 1/2014 |
FULL PRESCRIBING INFORMATION: CONTENTS*1 INDICATION AND USAGE2 DOSAGE AND ADMINISTRATION2.1 Individualization of Treatments2.2 Directions for use3 DOSAGE FORMS AND STRENGTHS4 CONTRAINDICATIONS5 WARNINGS AND PRECAUTIONS6 ADVERSE REACTIONS7 DRUG INTERACTIONS8 USE IN SPECIFIC POPULATION8.1 Pregnancy8.3 Nursing Mothers8.4 Pediatric8.5 Geriatric10 OVERDOSAGE11 DESCRIPTION12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action12.2 Pharmacokinetics16 HOW SUPPLIED STORAGE AND HANDLING
|
FULL PRESCRIBING INFORMATION
1 INDICATION AND USAGE
PrismaSol solution is indicated in adults and children for use as a replacement solution in Continuous Renal Replacement Therapy (CRRT) to replace plasma volume removed by ultrafiltration and to correct electrolytes and acid-base imbalances. PrismaSol solution may also be used in case of drug poisoning when CRRT is used to remove filterable substances.
2 DOSAGE AND ADMINISTRATION
2.1 Individualization of Treatments
The mode of therapy, solute formulation, flow rates and length of therapy should be selected by the physician responsible for managing treatment depending on the clinical condition of the patient as well as the patient's fluid, electrolyte, acid base and glucose balance. PrismaSol solution can be administered into the extra-corporeal circuit before (pre-dilution) and/or after the hemofilter or hemodiafilter (post-dilution).
In post-dilution hemofiltration, the replacement rate should not be greater than one third of the blood flow rate; e.g., for blood flow of 100 mL/min, equivalent to 6000 mL/hour, post-filter replacement rate should not exceed 2000 mL/hour.
2.2 Directions for use
PrismaSol solution should be inspected visually for particulate matter and discoloration prior to administration. Use only if the solution is clear and all seals, including the peel seal between the compartments, are intact. Press bag firmly to test for any leakage. Do not use if container is damaged or leaking.
When connecting solution bags, follow the instructions in this leaflet for correct use of the access ports. Incorrect use of the access port or other restrictions to fluid flow might lead to incorrect patient weight loss and may result in machine alarms. Continuing treatment without resolving the originating cause may result in patient injury or death.
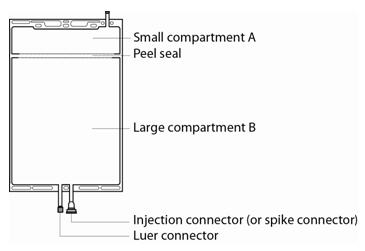
The electrolyte solution (small compartment A) is added to the buffer solution (large compartment B) by opening the peel seal immediately before use and mixing the contents of compartments A and B.
- The reconstituted solution is for single patient use only
- Aseptic technique should be used throughout administration to the patient.
- Discard any unused solution.
As soon as the overwrap is removed, the reconstitution of compartments A and B should be done and the reconstituted solution should be used immediately.
Due to chemical reasons, after removal of the overwrap, the solution is stable for 24 hours including the duration of the treatment.
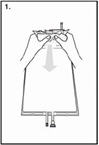 | Step 1 Immediately before use, remove the overwrap from the bag and mix the solutions in the two different compartments. Hold the small compartment with both hands and squeeze it until an opening is created in the peel seal. (See Figure 1 beside) |
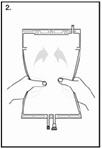 | Step 2 Squeeze with both hands on the large compartment until the peel seal between the two compartments is entirely open. Shake gently to mix. (See Figure 2 beside) The solution is now ready for use and can be hung on the equipment. |
Step 3 The replacement line may be connected to the bag through either of the luer connector or the injection connector (or spike connector).
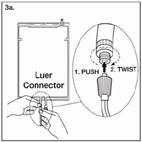 | Step 3a The luer connector is a needle-less and swabbable connector. Remove the cap with a twist and pull motion, and connect the male luer lock on the replacement line to the female luer receptor on the bag. (See Figure 3a beside) Ensure that the connection is fully seated and tighten. The connector is now open. Verify that the fluid is flowing freely during use. When the replacement line is disconnected from the luer connector, the connector will close and the flow of the solution will stop. |
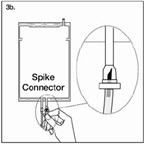 | Step 3b If the injection connector (or spike connector) is used, first remove the snap-off cap. Then introduce the replacement line spike through the rubber septum of the bag connector. (See Figure 3b beside) Ensure that the spike is fully inserted and verify that the fluid is flowing freely during use. |
Additions: The large compartment B is fitted with an injection connector (or spike connector) for the addition of drugs after reconstitution of the solution. When introducing additives, use aseptic techniques.
3 DOSAGE FORMS AND STRENGTHS
| BGK0/2.5 | BGK4/2.5 | BGK2/3.5 | BGK2/0 | B22GK4/0 | BK0/0/1.2 | BGK4/0/1.2 |
Each PrismaSol solution represents of a unique combination of the active ingredients listed below.
BEFORE RECONSTITUTION
1000 mL of clear electrolyte solution (small compartment A) contains (g):
| Active ingredients | PrismaSol BGK 0/2.5 | PrismaSol BGK 4/2.5 | PrismaSol BGK 2/3.5 | PrismaSol BGK 2/0 | PrismaSol B22GK 4/0 | PrismaSol BK 0/0/1.2 | PrismaSol BGK 4/0/1.2 |
|---|---|---|---|---|---|---|---|
| Calcium chloride • 2 H2O | 3.68 | 3.68 | 5.15 | 0 | 0 | 0 | 0 |
| Magnesium chloride • 6 H2O | 3.05 | 3.05 | 2.03 | 2.03 | 3.05 | 2.44 | 2.44 |
| Dextrose anhydrous | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | 0 | 20.0 |
| (as dextrose monohydrate) | 22.0 | 22.0 | 22.0 | 22.0 | 22.0 | 0 | 22.0 |
| Lactic acid | 5.40 | 5.40 | 5.40 | 5.40 | 5.40 | 5.40 | 5.40 |
1000 mL of buffer solution (large compartment B) contains (g):
| Active ingredients | PrismaSol BGK 0/2.5 | PrismaSol BGK 4/2.5 | PrismaSol BGK 2/3.5 | PrismaSol BGK 2/0 | PrismaSol B22GK 4/0 | PrismaSol BK 0/0/1.2 | PrismaSol BGK 4/0/1.2 |
|---|---|---|---|---|---|---|---|
| Sodium chloride | 6.46 | 6.46 | 6.46 | 6.46 | 7.07 | 6.46 | 6.46 |
| Sodium bicarbonate | 3.09 | 3.09 | 3.09 | 3.09 | 2.21 | 3.09 | 3.09 |
| Potassium chloride | 0 | 0.314 | 0.157 | 0.157 | 0.314 | 0 | 0.314 |
AFTER RECONSTITUTION OF COMPARTMENTS A AND B
1000 mL of the clear reconstituted solution contains:
| in mEq/L except where noted | PrismaSol BGK 0/2.5 | PrismaSol BGK 4/2.5 | PrismaSol BGK 2/3.5 | PrismaSol BGK 2/0 | PrismaSol B22GK 4/0 | PrismaSol BK 0/0/1.2 | PrismaSol BGK 4/0/1.2 |
|---|---|---|---|---|---|---|---|
| Calcium, Ca2+ | 2.5 | 2.5 | 3.5 | 0 | 0 | 0 | 0 |
| Bicarbonate, HCO3- | 32 | 32 | 32 | 32 | 22 | 32 | 32 |
| Potassium, K+ | 0 | 4.0 | 2.0 | 2.0 | 4.0 | 0 | 4.0 |
| Magnesium, Mg2+ | 1.5 | 1.5 | 1.0 | 1.0 | 1.5 | 1.2 | 1.2 |
| Sodium, Na+ | 140 | 140 | 140 | 140 | 140 | 140 | 140 |
| Chloride, Cl- | 109.0 | 113.0 | 111.5 | 108.0 | 120.5 | 106.2 | 110.2 |
| Lactate | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 |
| Dextrose | 100 mg/dL | 100 mg/dL | 100 mg/dL | 100 mg/dL | 100 mg/dL | 0 | 100 mg/dL |
| Theoretical Osmolarity | 292 mOsm/L | 300 mOsm/L | 296 mOsm/L | 291 mOsm/L | 296 mOsm/L | 282 mOsm/L | 295 mOsm/L |
5 WARNINGS AND PRECAUTIONS
The electrolyte solution contained in compartment A must be mixed with the buffer solution of compartment B before use in order to obtain the reconstituted solution suitable for hemofiltration /hemodiafiltration.
Due to chemical reasons, after removal of the overwrap, the solution is stable for 24 hours including the duration of the treatment.
Do not administer the reconstituted solution unless it is clear and free of visible particulate matter.
PrismaSol solution includes several formulations. Selection of a specific formulation depends on the patient's condition and treatment procedures.
Administration of the solution should only be under the direction of a physician competent in intensive care treatment including CRRT.
The patient's hemodynamic fluid, electrolyte and acid-base balance should be monitored throughout the procedure. Note that citrate, when used as an anticoagulant, contributes to the base load and can reduce plasma calcium levels.
During hemofiltration or hemodiafiltration, abnormalities in the plasma concentration of potassium, calcium, and glucose may develop. These abnormalities may be corrected by the use of appropriate formulations of PrismaSol solution. Abnormalities in plasma phosphate concentration, especially hypophosphatemia, may also occur. Hypophosphatemia may require phosphate supplementation to maintain plasma concentrations in the physiologic range.
Use only with continuous extra-corporeal blood purification equipment in CRRT.
When connecting solution bags, follow the instructions in this leaflet for correct use of the access ports. Incorrect use of the access port or other restrictions to fluid flow might lead to incorrect patient weight loss and may result in machine alarms. Continuing treatment without resolving the originating cause may result in patient injury or death.
The solution may be heated to no more than 40°C/104°F inside of the overwrap and this must be carefully controlled. After heating, verify that the solution remains clear and contains no particulate matter.
6 ADVERSE REACTIONS
Adverse reactions can result from the solution or the CRRT procedure.
Improper use can lead to fluid imbalance and disturbances in electrolyte, acid-base and glucose balance.
7 DRUG INTERACTIONS
As with the use of other replacement solutions, blood concentrations of filterable drugs may be influenced by CRRT.
The blood concentrations of certain drugs may need to be monitored and appropriate therapy implemented to correct for removal during treatment. In patients with cardiovascular disease, especially those using cardiac glycoside medications, plasma levels of calcium, potassium and magnesium must be carefully monitored.
8 USE IN SPECIFIC POPULATION
8.1 Pregnancy
Pregnancy Category C.
PrismaSol solution is a replacement solution of electrolytes, bicarbonate and dextrose and is pharmacologically inactive. Animal reproduction studies have not been conducted with PrismaSol solution. While there are no adequate and well controlled studies in pregnant women, appropriate administration of PrismaSol solution with monitoring of fluid, electrolyte, acid-base and glucose balance, is not expected to cause fetal harm, or affect reproductive capacity. Maintenance of normal acid-base balance is important for fetal well being.
8.3 Nursing Mothers
PrismaSol solution is a replacement solution of electrolytes, bicarbonate and dextrose and is pharmacologically inactive. The components of PrismaSol solution are excreted in human milk. Appropriate administration of PrismaSol solution with monitoring of fluid, electrolyte, acid-base and glucose balance, is not expected to harm a nursing infant.
8.4 Pediatric
Safety and effectiveness have been established based on published clinical data of CCRT replacement solutions used in adults and children. Two hemofiltration studies have shown that CRRT can be used to treat pediatric patients, including a study of newborns to 17 years olds. These pediatric studies utilized replacement solutions with a composition similar to PrismaSol Solutions. No adequate and well-controlled studies have been conducted with PrismaSol Solutions in pediatric patients.
8.5 Geriatric
Safety and effectiveness in geriatric patients have been reported in some studies on the general population. No formal specific study was carried out in the geriatric population.
Although clinical experience has not identified differences in responses between elderly and younger patients, greater sensitivity of some individuals cannot be ruled out.
10 OVERDOSAGE
Overdosage with PrismaSol solution should not occur if the procedure is carried out appropriately with careful monitoring of fluid, electrolyte, acid-base and glucose balance. However, overdosage can occur if fluid administration is in excess of the volume required to achieve the prescribed fluid balance or if delivery of a solute component in the solution exceeds a patient's needs. On the other hand, excessive fluid and solute removal may also occur in CRRT, potentially resulting in volume depletion and solute deficits.
The management of either an excess or deficiency state with respect to fluid, electrolyte and acid-base balance may involve modifications in the rate of administration and/or the composition of CRRT solutions. In conjunction with these changes, adjustments in the effluent (dialysate or ultrafiltrate) rate may need to occur.
11 DESCRIPTION
PrismaSol solution is a clear, sterile solution free of bacterial endotoxins. This solution is used in Continuous Renal Replacement Therapies (CRRT) as a replacement solution in hemofiltration and hemodiafiltration.
It contains no bacteriostatic or antimicrobial agents.
PrismaSol solution is packaged in a two-compartment bag. The small compartment A contains electrolytes and the large compartment B contains buffer. The final reconstituted solution (5000 mL) is obtained after breaking the peel seal between compartment A and B and mixing both solutions.
Calcium chloride, USP, is chemically designated calcium chloride dihydrate (CaCl2 • 2 H2O).
Magnesium chloride, USP, is chemically designated magnesium chloride hexahydrate (MgCl2 • 6 H2O).
Dextrose, USP, is chemically designated D-Glucose anhydrous (C6H12O6) or D-Glucose monohydrate (C6H12O6 • H2O).
Lactic acid, USP, is chemically designated CH3CH(OH)COOH.
Sodium chloride, USP, is chemically designated NaCl.
Potassium chloride, USP, is chemically designated KCl.
Sodium bicarbonate, USP, is chemically designated NaHCO3.
The pH of the final solution is in the range of 7.0 to 8.5.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
PrismaSol solution is a pharmacologically inactive solution. The electrolyte concentrations in the PrismaSol solutions are chosen to restore plasma levels to clinically desired concentrations or maintain plasma levels at the desired concentrations.
PrismaSol solution is used as replacement solution to replace water and electrolytes removed during hemofiltration and hemodiafiltration. Bicarbonate in the solution is used as an alkalinizing buffer to normalize acid-base balance.Lactate is used for the adjustment of the solution pH and is metabolized to bicarbonate.
When dextrose is present, it is intended to help normalize glucose balance.
12.2 Pharmacokinetics
The distribution of electrolytes, bicarbonate and dextrose is determined by the patient's clinical condition, metabolic status, and residual renal function.
The elimination and replacement of water, electrolytes and buffer depend on the patient's electrolyte and acid-base balance, metabolic status, residual renal function and ongoing physiologic losses through intestinal, respiratory and cutaneous routes.
16 HOW SUPPLIED STORAGE AND HANDLING
PrismaSol solution is supplied in a two compartment bag made of polyolefin. Polyolefin does not contain the chemical component di-2-ethyl hexyl phthalate (DEHP).
The 5000 mL bag is composed of a small compartment (250 mL) and a large compartment (4750 mL). The two compartments are separated by a peel seal.
The bag is overwrapped with a transparent overwrap.
| Container | Fill Volume | NDC |
|---|---|---|
| PrismaSol BGK0/2.5 | 5000 mL | 24571-108-06 |
| PrismaSol BGK4/2.5 | 5000 mL | 24571-105-06 |
| PrismaSol BGK2/3.5 | 5000 mL | 24571-103-06 |
| PrismaSol BGK2/0 | 5000 mL | 24571-102-06 |
| PrismaSol B22GK4/0 | 5000 mL | 24571-111-06 |
| PrismaSol BK0/0/1.2 | 5000 mL | 24571-113-06 |
| PrismaSol BGK4/0/1.2 | 5000 mL | 24571-114-06 |
Not all formulations may be marketed.
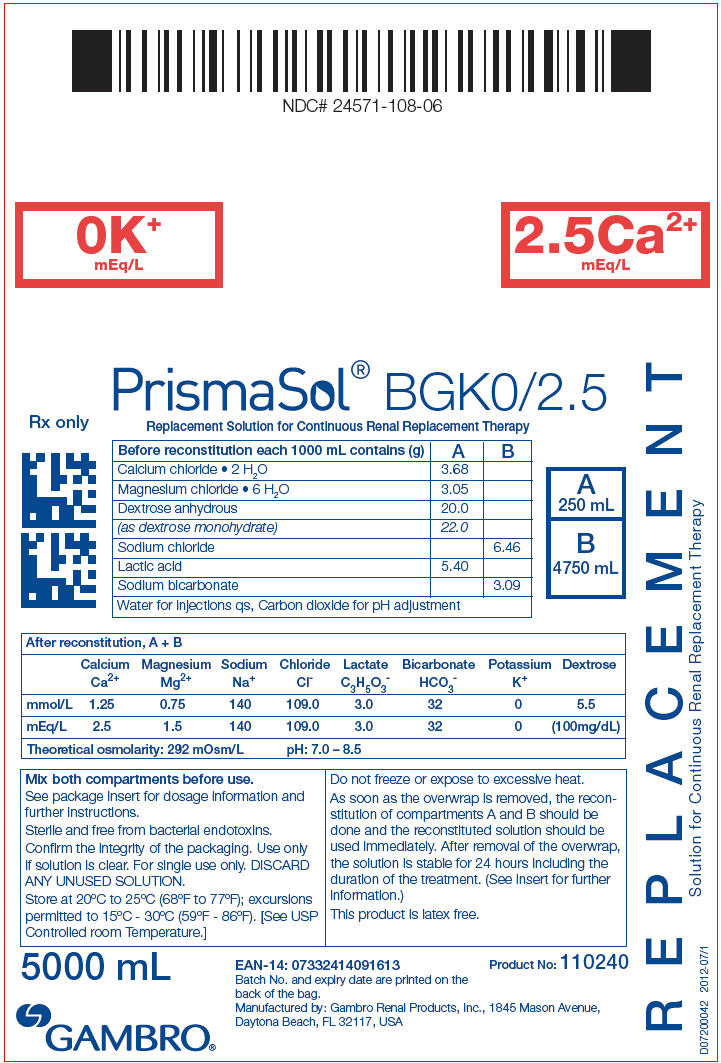
PRINCIPAL DISPLAY PANEL - BGK0/2.5 Bag Label
NDC# 24571-108-06
0K+
mEq/L
2.5Ca2+
mEq/L
PrismaSol® BGK0/2.5
Replacement Solution for Continuous Renal Replacement Therapy
Rx only
A
250 mL
B
4750 mL
| Before reconstitution each 1000 mL contains (g) | A | B |
|---|---|---|
| Calcium chloride • 2 H2O | 3.68 | |
| Magnesium chloride • 6 H2O | 3.05 | |
| Dextrose anhydrous | 20.0 | |
| (as dextrose monohydrate) | 22.0 | |
| Sodium chloride | 6.46 | |
| Lactic acid | 5.40 | |
| Sodium bicarbonate | 3.09 | |
| Water for injections qs, Carbon dioxide for pH adjustment | ||
| After reconstitution, A + B | ||||||||
|---|---|---|---|---|---|---|---|---|
| Calcium Ca2+ | Magnesium Mg2+ | Sodium Na+ | Chloride Cl- | Lactate C3H5O3- | Bicarbonate HCO3- | Potassium K+ | Dextrose | |
| mmol/L | 1.25 | 0.75 | 140 | 109.0 | 3.0 | 32 | 0 | 5.5 |
| mEq/L | 2.5 | 1.5 | 140 | 109.0 | 3.0 | 32 | 0 | (100mg/dL) |
| Theoretical osmolarity: 292 mOsm/L | pH: 7.0 – 8.5 | |||||||
Mix both compartments before use.
See package insert for dosage information and
further instructions.
Sterile and free from bacterial endotoxins.
Confirm the integrity of the packaging. Use only
if solution is clear. For single use only. DISCARD
ANY UNUSED SOLUTION.
Store at 20°C to 25°C (68°F to 77°F); excursions
permitted to 15°C - 30°C (59°F - 86°F). [See USP
Controlled room Temperature.]
Do not freeze or expose to excessive heat.
As soon as the overwrap is removed, the recon-
stitution of compartments A and B should be
done and the reconstituted solution should be
used immediately. After removal of the overwrap,
the solution is stable for 24 hours including the
duration of the treatment. (See insert for further
information.)
This product is latex free.
5000 mL
GAMBRO®
EAN-14: 07332414091613
Batch No. and expiry date are printed on the
back of the bag.
Manufactured by: Gambro Renal Products, Inc., 1845 Mason Avenue,
Daytona Beach, FL 32117, USA
Product No: 110240
R E P L A C E M E N T
Solution for Continuous Renal Replacement Therapy
D07200042 2012-07/1
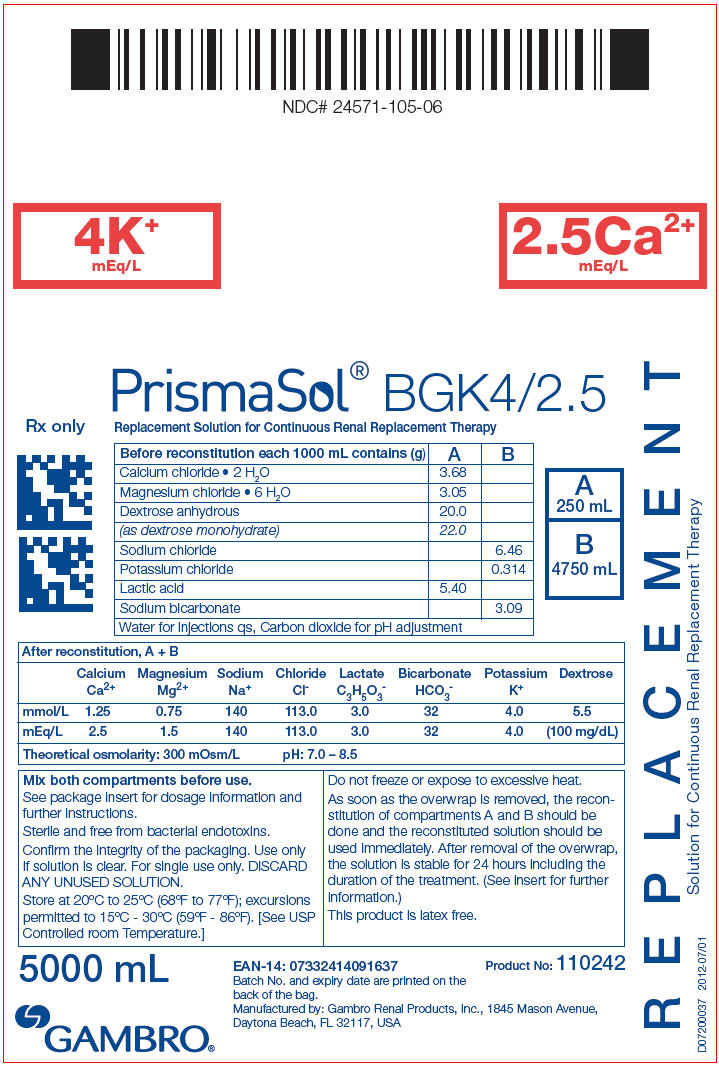
PRINCIPAL DISPLAY PANEL - BGK4/2.5 Bag Label
NDC# 24571-105-06
4K+
mEq/L
2.5Ca2+
mEq/L
PrismaSol® BGK4/2.5
Replacement Solution for Continuous Renal Replacement Therapy
Rx only
A
250 mL
B
4750 mL
| Before reconstitution each 1000 mL contains (g) | A | B |
|---|---|---|
| Calcium chloride • 2 H2O | 3.68 | |
| Magnesium chloride • 6 H2O | 3.05 | |
| Dextrose anhydrous | 20.0 | |
| (as dextrose monohydrate) | 22.0 | |
| Sodium chloride | 6.46 | |
| Potassium chloride | 0.314 | |
| Lactic acid | 5.40 | |
| Sodium bicarbonate | 3.09 | |
| Water for Injections qs, Carbon dioxide for pH adjustment | ||
| After reconstitution, A + B | ||||||||
|---|---|---|---|---|---|---|---|---|
| Calcium Ca2+ | Magnesium Mg2+ | Sodium Na+ | Chloride Cl- | Lactate C3H5O3- | Bicarbonate HCO3- | Potassium K+ | Dextrose | |
| mmol/L | 1.25 | 0.75 | 140 | 113.0 | 3.0 | 32 | 4.0 | 5.5 |
| mEq/L | 2.5 | 1.5 | 140 | 113.0 | 3.0 | 32 | 4.0 | (100 mg/dL) |
| Theoretical osmolarity: 300 mOsm/L | pH: 7.0 – 8.5 | |||||||
Mix both compartments before use.
See package insert for dosage information and
further instructions.
Sterile and free from bacterial endotoxins.
Confirm the integrity of the packaging. Use only
if solution is clear. For single use only. DISCARD
ANY UNUSED SOLUTION.
Store at 20°C to 25°C (68°F to 77°F); excursions
permitted to 15°C - 30°C (59°F - 86°F). [See USP
Controlled room Temperature.]
Do not freeze or expose to excessive heat.
As soon as the overwrap is removed, the recon-
stitution of compartments A and B should be
done and the reconstituted solution should be
used immediately. After removal of the overwrap,
the solution is stable for 24 hours including the
duration of the treatment. (See insert for further
information.)
This product is latex free.
5000 mL
GAMBRO®
EAN-14: 07332414091637
Batch No. and expiry date are printed on the
back of the bag.
Manufactured by: Gambro Renal Products, Inc., 1845 Mason Avenue,
Daytona Beach, FL 32117, USA
Product No: 110242
R E P L A C E M E N T
Solution for Continuous Renal Replacement Therapy
D07200037 2012-07/01
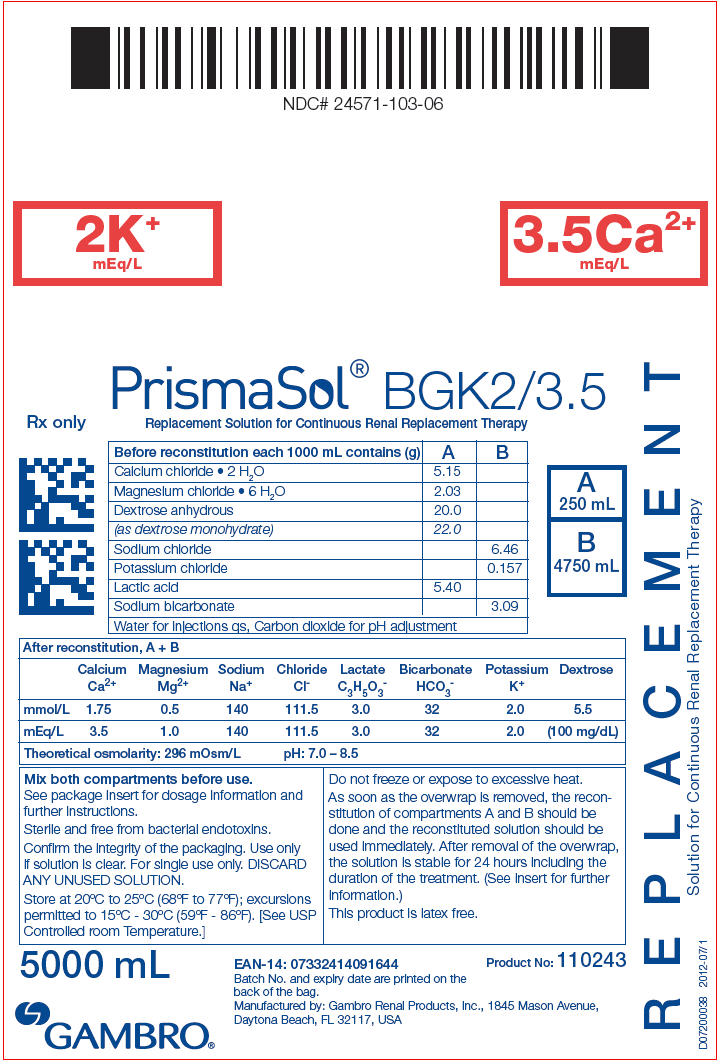
PRINCIPAL DISPLAY PANEL - BGK2/3.5 Bag Label
NDC# 24571-103-06
2K+
mEq/L
3.5Ca2+
mEq/L
PrismaSol® BGK2/3.5
Replacement Solution for Continuous Renal Replacement Therapy
Rx only
A
250 mL
B
4750 mL
| Before reconstitution each 1000 mL contains (g) | A | B |
|---|---|---|
| Calcium chloride • 2 H2O | 5.15 | |
| Magnesium chloride • 6 H2O | 2.03 | |
| Dextrose anhydrous | 20.0 | |
| (as dextrose monohydrate) | 22.0 | |
| Sodium chloride | 6.46 | |
| Potassium chloride | 0.157 | |
| Lactic acid | 5.40 | |
| Sodium bicarbonate | 3.09 | |
| Water for injections qs, Carbon dioxide for pH adjustment | ||
| After reconstitution, A + B | ||||||||
|---|---|---|---|---|---|---|---|---|
| Calcium Ca2+ | Magnesium Mg2+ | Sodium Na+ | Chloride Cl- | Lactate C3H5O3- | Bicarbonate HCO3- | Potassium K+ | Dextrose | |
| mmol/L | 1.75 | 0.5 | 140 | 111.5 | 3.0 | 32 | 2.0 | 5.5 |
| mEq/L | 3.5 | 1.0 | 140 | 111.5 | 3.0 | 32 | 2.0 | (100 mg/dL) |
| Theoretical osmolarity: 296 mOsm/L | pH: 7.0 – 8.5 | |||||||
Mix both compartments before use.
See package insert for dosage information and
further instructions.
Sterile and free from bacterial endotoxins.
Confirm the integrity of the packaging. Use only
if solution is clear. For single use only. DISCARD
ANY UNUSED SOLUTION.
Store at 20°C to 25°C (68°F to 77°F); excursions
permitted to 15°C - 30°C (59°F - 86°F). [See USP
Controlled room Temperature.]
Do not freeze or expose to excessive heat.
As soon as the overwrap is removed, the recon-
stitution of compartments A and B should be
done and the reconstituted solution should be
used immediately. After removal of the overwrap,
the solution is stable for 24 hours including the
duration of the treatment. (See insert for further
information.)
This product is latex free.
5000 mL
GAMBRO®
EAN-14: 07332414091644
Batch No. and expiry date are printed on the
back of the bag.
Manufactured by: Gambro Renal Products, Inc., 1845 Mason Avenue,
Daytona Beach, FL 32117, USA
Product No: 110243
R E P L A C E M E N T
Solution for Continuous Renal Replacement Therapy
D07200038 2012-07/1
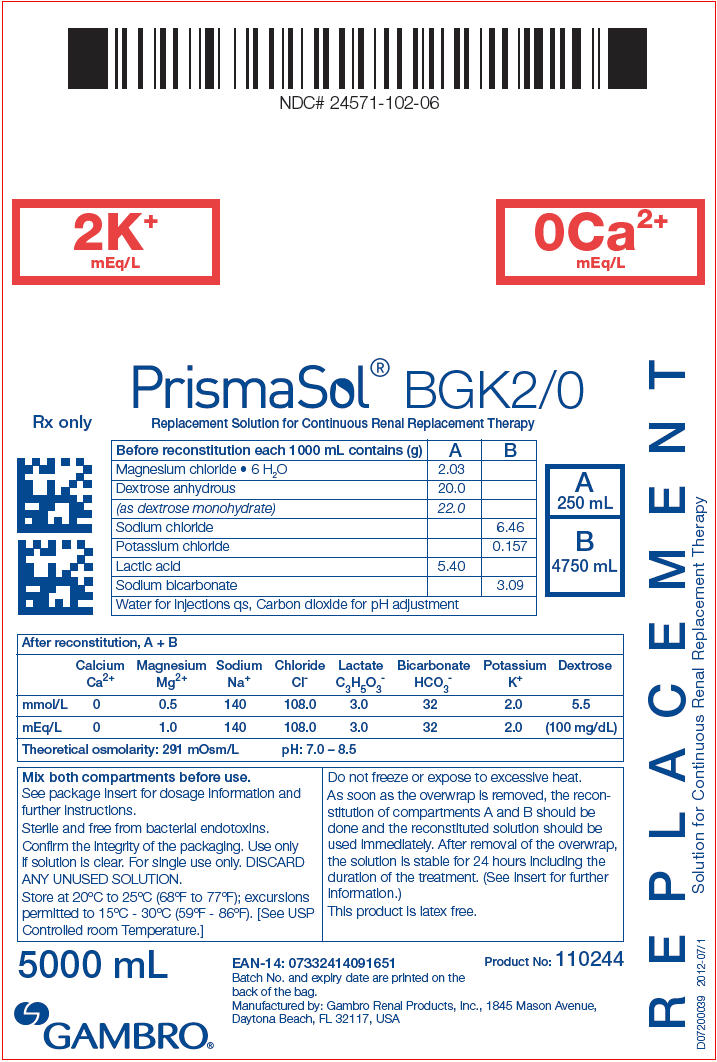
PRINCIPAL DISPLAY PANEL - BGK2/0 Bag Label
NDC# 24571-102-06
2K+
mEq/L
0Ca2+
mEq/L
PrismaSol® BGK2/0
Replacement Solution for Continuous Renal Replacement Therapy
Rx only
A
250 mL
B
4750 mL
| Before reconstitution each 1000 mL contains (g) | A | B |
|---|---|---|
| Magnesium chloride • 6 H2O | 2.03 | |
| Dextrose anhydrous | 20.0 | |
| (as dextrose monohydrate) | 22.0 | |
| Sodium chloride | 6.46 | |
| Potassium chloride | 0.157 | |
| Lactic acid | 5.40 | |
| Sodium bicarbonate | 3.09 | |
| Water for injections qs, Carbon dioxide for pH adjustment | ||
| After reconstitution, A + B | ||||||||
|---|---|---|---|---|---|---|---|---|
| Calcium Ca2+ | Magnesium Mg2+ | Sodium Na+ | Chloride Cl- | Lactate C3H5O3- | Bicarbonate HCO3- | Potassium K+ | Dextrose | |
| mmol/L | 0 | 0.5 | 140 | 108.0 | 3.0 | 32 | 2.0 | 5.5 |
| mEq/L | 0 | 1.0 | 140 | 108.0 | 3.0 | 32 | 2.0 | (100 mg/dL) |
| Theoretical osmolarity: 291 mOsm/L | pH: 7.0 – 8.5 | |||||||
Mix both compartments before use.
See package insert for dosage information and
further instructions.
Sterile and free from bacterial endotoxins.
Confirm the integrity of the packaging. Use only
if solution is clear. For single use only. DISCARD
ANY UNUSED SOLUTION.
Store at 20°C to 25°C (68°F to 77°F); excursions
permitted to 15°C - 30°C (59°F - 86°F). [See USP
Controlled room Temperature.]
Do not freeze or expose to excessive heat.
As soon as the overwrap is removed, the recon-
stitution of compartments A and B should be
done and the reconstituted solution should be
used immediately. After removal of the overwrap,
the solution is stable for 24 hours including the
duration of the treatment. (See insert for further
information.)
This product is latex free.
5000 mL
GAMBRO®
EAN-14: 07332414091651
Batch No. and expiry date are printed on the
back of the bag.
Manufactured by: Gambro Renal Products, Inc., 1845 Mason Avenue,
Daytona Beach, FL 32117, USA
Product No: 110244
R E P L A C E M E N T
Solution for Continuous Renal Replacement Therapy
D07200039 2012-07/1
PRINCIPAL DISPLAY PANEL - B22GK4/0 Bag Label
NDC# 24571-111-06
4K+
mEq/L
Bicarbonate 22
0Ca2+
mEq/L
PrismaSol® B22GK4/0
Replacement Solution for Continuous Renal Replacement Therapy
Rx only
A
250 mL
B
4750 mL
| Before reconstitution each 1000 mL contains (g) | A | B |
|---|---|---|
| Magnesium chloride • 6 H2O | 3.05 | |
| Dextrose anhydrous | 20.0 | |
| (as dextrose monohydrate) | 22.0 | |
| Sodium chloride | 7.07 | |
| Potassium chloride | 0.314 | |
| Lactic acid | 5.40 | |
| Sodium bicarbonate | 2.21 | |
| Water for injections qs, Carbon dioxide for pH adjustment | ||
| After reconstitution, A + B | ||||||||
|---|---|---|---|---|---|---|---|---|
| Calcium Ca2+ | Magnesium Mg2+ | Sodium Na+ | Chloride Cl- | Lactate C3H5O3- | Bicarbonate HCO3- | Potassium K+ | Dextrose | |
| mmol/L | 0 | 0.75 | 140 | 120.5 | 3.0 | 22 | 4.0 | 5.5 |
| mEq/L | 0 | 1.5 | 140 | 120.5 | 3.0 | 22 | 4.0 | (100mg/dL) |
| Theoretical osmolarity: 296 mOsm/L | pH: 7.0 – 8.5 | |||||||
Mix both compartments before use.
See package insert for dosage information and
further instructions.
Sterile and free from bacterial endotoxins.
Confirm the integrity of the packaging. Use only
if solution is clear. For single use only. DISCARD
ANY UNUSED SOLUTION.
Store at 20°C to 25°C (68°F to 77°F); excursions
permitted to 15°C - 30°C (59°F - 86°F). [See USP
Controlled room Temperature.]
Do not freeze or expose to excessive heat.
As soon as the overwrap is removed, the recon-
stitution of compartments A and B should be
done and the reconstituted solution should be
used immediately. After removal of the overwrap,
the solution is stable for 24 hours including the
duration of the treatment. (See insert for further
information.)
This product is latex free.
5000 mL
GAMBRO®
EAN-14: 07332414116781
Batch No. and expiry date are printed on the
back of the bag.
Manufactured by: Gambro Renal Products, Inc., 1845 Mason Avenue,
Daytona Beach, FL 32117, USA
Product No: 115001
R E P L A C E M E N T
Solution for Continuous Renal Replacement Therapy
D11000102 2012-07/1

PRINCIPAL DISPLAY PANEL - BK0/0/1.2 Bag Label
NDC# 24571-113-06
0K+
mEq/L
0Ca2+
mEq/L
PrismaSol® BGK0/0/1.2
Replacement Solution for Continuous Renal Replacement Therapy
Rx only
A
250 mL
B
4750 mL
| Before reconstitution each 1000 mL contains (g) | A | B |
|---|---|---|
| Magnesium chloride • 6 H2O | 2.44 | |
| Sodium chloride | 6.46 | |
| Lactic acid | 5.40 | |
| Sodium bicarbonate | 3.09 | |
| Water for injections qs, Carbon dioxide for pH adjustment | ||
| After reconstitution, A + B | ||||||||
|---|---|---|---|---|---|---|---|---|
| Calcium Ca2+ | Magnesium Mg2+ | Sodium Na+ | Chloride Cl- | Lactate C3H5O3- | Bicarbonate HCO3- | Potassium K+ | Dextrose | |
| mmol/L | 0 | 0.6 | 140 | 106.2 | 3.0 | 32 | 0 | 0 |
| mEq/L | 0 | 1.2 | 140 | 106.2 | 3.0 | 32 | 0 | (0mg/dL) |
| Theoretical osmolarity: 282 mOsm/L | pH: 7.0 – 8.5 | |||||||
Mix both compartments before use.
See package insert for dosage information and
further instructions.
Sterile and free from bacterial endotoxins.
Confirm the integrity of the packaging. Use only
if solution is clear. For single use only. DISCARD
ANY UNUSED SOLUTION.
Store at 20°C to 25°C (68°F to 77°F); excursions
permitted to 15°C - 30°C (59°F - 86°F). [See USP
Controlled room Temperature.]
Do not freeze or expose to excessive heat.
As soon as the overwrap is removed, the recon-
stitution of compartments A and B should be
done and the reconstituted solution should be
used immediately. After removal of the overwrap,
the solution is stable for 24 hours including the
duration of the treatment. (See insert for further
information.)
This product is latex free.
5000 mL
GAMBRO®
EAN-14: 07332414091309
Batch No. and expiry date are printed on the
back of the bag.
Manufactured by: Gambro Renal Products, Inc., 1845 Mason Avenue,
Daytona Beach, FL 32117, USA
Product No: 110239
R E P L A C E M E N T
Solution for Continuous Renal Replacement Therapy
D07200041 2012-07/1

PRINCIPAL DISPLAY PANEL - BGK4/0/1.2 Bag Label
NDC# 24571-114-06
4K+
mEq/L
0Ca2+
mEq/L
PrismaSol® BGK4/0/1.2
Replacement Solution for Continuous Renal Replacement Therapy
Rx only
A
250 mL
B
4750 mL
| Before reconstitution each 1000 mL contains (g) | A | B |
|---|---|---|
| Magnesium chloride • 6 H2O | 2.44 | |
| Dextrose anhydrous | 20.0 | |
| (as dextrose monohydrate) | 22.0 | |
| Sodium chloride | 6.46 | |
| Potassium chloride | 0.314 | |
| Lactic acid | 5.40 | |
| Sodium bicarbonate | 3.09 | |
| Water for injections qs, Carbon dioxide for pH adjustment | ||
| After reconstitution, A + B | ||||||||
|---|---|---|---|---|---|---|---|---|
| Calcium Ca2+ | Magnesium Mg2+ | Sodium Na+ | Chloride Cl- | Lactate C3H5O3- | Bicarbonate HCO3- | Potassium K+ | Dextrose | |
| mmol/L | 0 | 0.6 | 140 | 110.2 | 3.0 | 32 | 4.0 | 5.5 |
| mEq/L | 0 | 1.2 | 140 | 110.2 | 3.0 | 32 | 4.0 | (100 mg/dL) |
| Theoretical osmolarity: 295 mOsm/L | pH: 7.0 – 8.5 | |||||||
Mix both compartments before use.
See package insert for dosage information and
further instructions.
Sterile and free from bacterial endotoxins.
Confirm the integrity of the packaging. Use only
if solution is clear. For single use only. DISCARD
ANY UNUSED SOLUTION.
Store at 20°C to 25°C (68°F to 77°F); excursions
permitted to 15°C - 30°C (59°F - 86°F). [See USP
Controlled room Temperature.]
Do not freeze or expose to excessive heat.
As soon as the overwrap is removed, the recon-
stitution of compartments A and B should be
done and the reconstituted solution should be
used immediately. After removal of the overwrap,
the solution is stable for 24 hours including the
duration of the treatment. (See insert for further
information.)
This product is latex free.
5000 mL
GAMBRO®
EAN-14: 07332414091620
Batch No. and expiry date are printed on the
back of the bag.
Manufactured by: Gambro Renal Products, Inc., 1845 Mason Avenue,
Daytona Beach, FL 32117, USA
Product No: 110241
R E P L A C E M E N T
Solution for Continuous Renal Replacement Therapy
D07200043 2012-07/1

| PRISMASOL
BGK0/2.5
calcium chloride, magnesium chloride, dextrose monohydrate, lactic acid, sodium chloride, and sodium bicarbonate solution |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| PRISMASOL
BGK4/2.5
calcium chloride, magnesium chloride, dextrose monohydrate, lactic acid, sodium chloride, sodium bicarbonate, and potassium chloride solution |
|||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||
| PRISMASOL
BGK2/3.5
calcium chloride, magnesium chloride, dextrose monohydrate, lactic acid, sodium chloride, sodium bicarbonate, and potassium chloride solution |
|||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||
| PRISMASOL
BGK2/0
magnesium chloride, dextrose monohydrate, lactic acid, sodium chloride, sodium bicarbonate, and potassium chloride solution |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| PRISMASOL
B22GK4/0
magnesium chloride, dextrose monohydrate, lactic acid, sodium chloride, sodium bicarbonate, and potassium chloride solution |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| PRISMASOL
BK0/0/1.2
magnesium chloride, lactic acid, sodium chloride, and sodium bicarbonate solution |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| PRISMASOL
BGK4/0/1.2
magnesium chloride, dextrose monohydrate, lactic acid, sodium chloride, sodium bicarbonate, and potassium chloride solution |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Gambro Renal Products (041093923) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Gambro Renal Products | 041093923 | MANUFACTURE(24571-102, 24571-103, 24571-105, 24571-108, 24571-111, 24571-113, 24571-114) | |