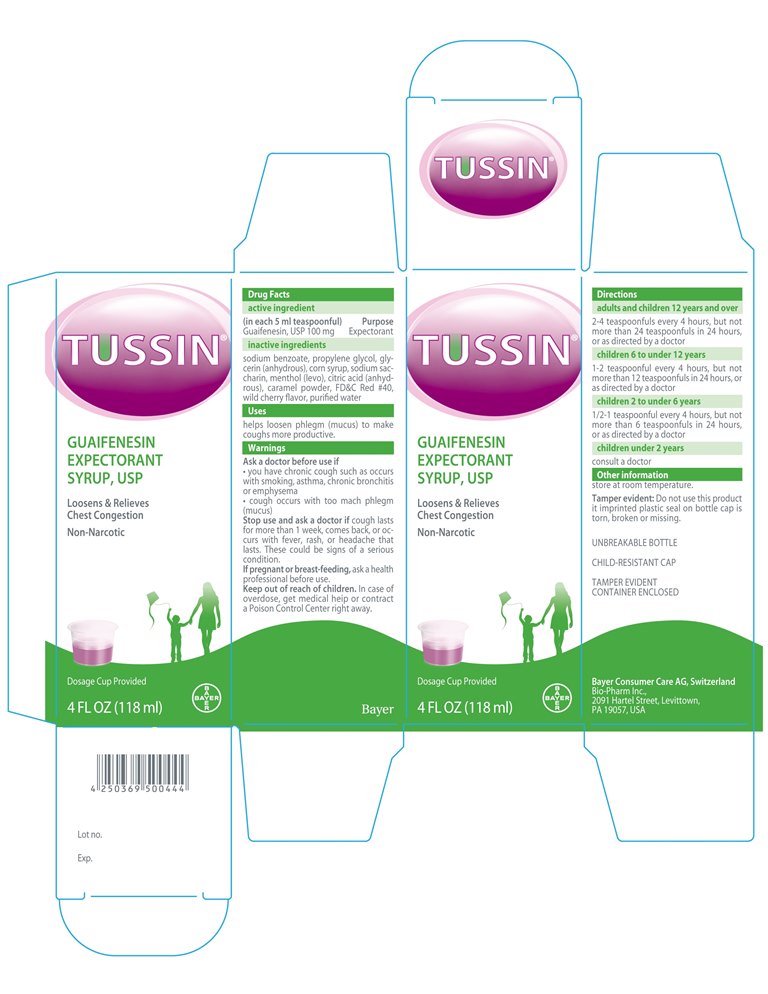
Label: TUSSIN GUIFENESIN EXPECTRORANT SYRUP- guifenesin syrup
-
Contains inactivated NDC Code(s)
NDC Code(s): 61841-403-26 - Packager: Bayer HealthCare LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Export only
Drug Label Information
Updated January 14, 2015
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredients (in each 5ml teaspoonful)
- Purpose
- INDICATIONS & USAGE
- WARNINGS
- ASK DOCTOR
- ASK DOCTOR/PHARMACIST
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- PRINCIPAL DISPLAY PANEL
- DOSAGE & ADMINISTRATION
- INACTIVE INGREDIENT
-
INGREDIENTS AND APPEARANCE
TUSSIN GUIFENESIN EXPECTRORANT SYRUP
guifenesin syrupProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:61841-403 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 100 mg in 5 mL Inactive Ingredients Ingredient Name Strength SODIUM BENZOATE (UNII: OJ245FE5EU) SACCHARIN SODIUM (UNII: SB8ZUX40TY) MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) GLYCERIN (UNII: PDC6A3C0OX) CORN SYRUP (UNII: 9G5L16BK6N) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) FD&C RED NO. 40 (UNII: WZB9127XOA) WATER (UNII: 059QF0KO0R) Product Characteristics Color pink Score Shape Size Flavor CHERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61841-403-26 1 in 1 CARTON 01/14/2015 1 118 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 01/14/2015 Labeler - Bayer HealthCare LLC (785159372) Establishment Name Address ID/FEI Business Operations Bio-Pharm, Inc. 801652546 manufacture(61841-403) , pack(61841-403)