EQUALINE TUSSIN CHEST CONGESTION- guaifenesin syrup
Supervalu Inc
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
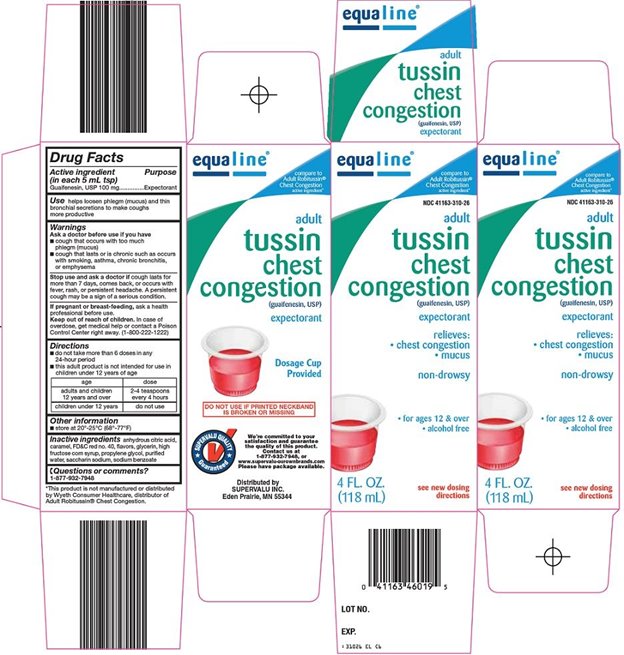
Supervalu Tussin Chest Congestion Drug Facts
Warnings
Ask a doctor before use if you have
- •
- cough that occurs with too much phlegm (mucus)
- •
- cough that lasts or is chronic such as occurs with smoking, asthma, chronic bronchitis, or emphysema
Directions
- •
- do not take more than 6 doses in any 24-hour period
- •
- this adult product is not intended for use in children under 12 years of age
|
age |
dose |
|
adults and children 12 years and over |
2-4 teaspoons every 4 hours |
|
children under 12 years |
do not use |
Inactive ingredients
anhydrous citric acid, caramel, FD&C red no. 40, flavors, glycerin, high fructose corn syrup, propylene glycol, purified water, saccharin sodium, sodium benzoate
| EQUALINE TUSSIN
CHEST CONGESTION
guaifenesin syrup |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Supervalu Inc (006961411) |
Revised: 11/2017
Document Id: 8ea4f01a-ada3-4fb9-ba73-d80366c5a40e
Set id: 0c3e8ce0-b4ff-4e74-a34c-477766864578
Version: 2
Effective Time: 20171126
Supervalu Inc