Label: KISS FUNGI-GONE ANTI-FUNGAL TREATMENT- tolnaftate liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 42432-101-03, 42432-101-04, 42432-101-05, 42432-101-06, view more42432-101-11, 42432-101-51 - Packager: Kiss Products, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated February 25, 2015
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
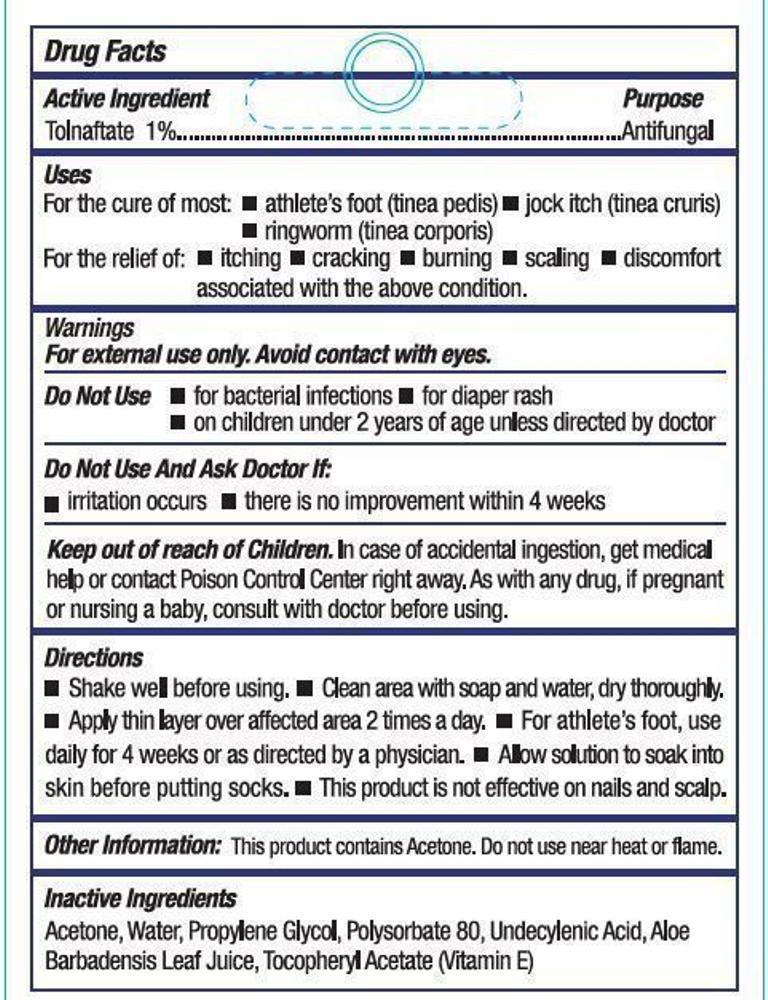
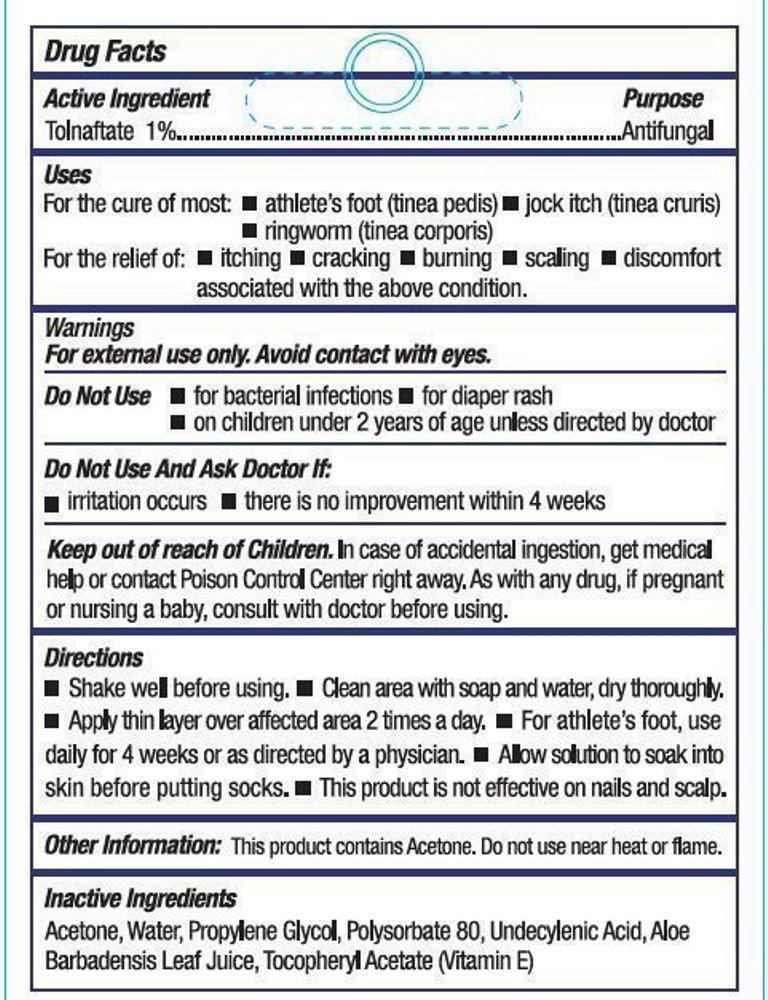
- Active Ingredient
- Purpose
- Uses
- Warnings
- Do not use
- Do not use and ask doctor if:
- KEEP OUT OF REACH OF CHILDREN.
-
Directions
- Shake well before using
- Clean area with soap and water, dry thoroughly
- Apply thin layer over affected area 2 times a day
- For athlete's foot, use daily for 4 weeks or as directed by a physician
- Allow solution to soak into the skin before putting socks on
- This product is not effective on nails and scalp
- Other Information
- Inactive Ingredients
- Image of Labels and Cartons
-
INGREDIENTS AND APPEARANCE
KISS FUNGI-GONE ANTI-FUNGAL TREATMENT
tolnaftate liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:42432-101 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TOLNAFTATE (UNII: 06KB629TKV) (TOLNAFTATE - UNII:06KB629TKV) TOLNAFTATE 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength ACETONE (UNII: 1364PS73AF) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) POLYSORBATE 80 (UNII: 6OZP39ZG8H) UNDECYLENIC ACID (UNII: K3D86KJ24N) ALOE VERA LEAF (UNII: ZY81Z83H0X) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:42432-101-04 1 in 1 BOX 1 NDC:42432-101-03 15 mL in 1 BOTTLE, WITH APPLICATOR 2 NDC:42432-101-51 1 in 1 BOX 2 NDC:42432-101-11 15 mL in 1 BOTTLE, WITH APPLICATOR 3 NDC:42432-101-06 1 in 1 BOX 3 NDC:42432-101-05 30 mL in 1 BOTTLE, WITH APPLICATOR Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333C 10/23/2012 Labeler - Kiss Products, Inc. (626892020)


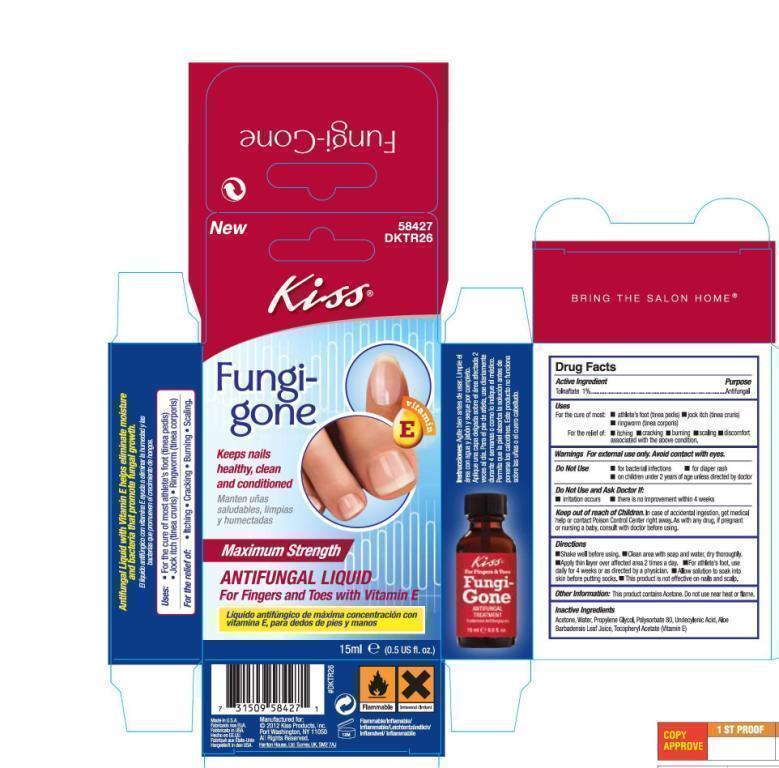


 FungiGoneHalfOunceCarton.jpg
FungiGoneHalfOunceCarton.jpg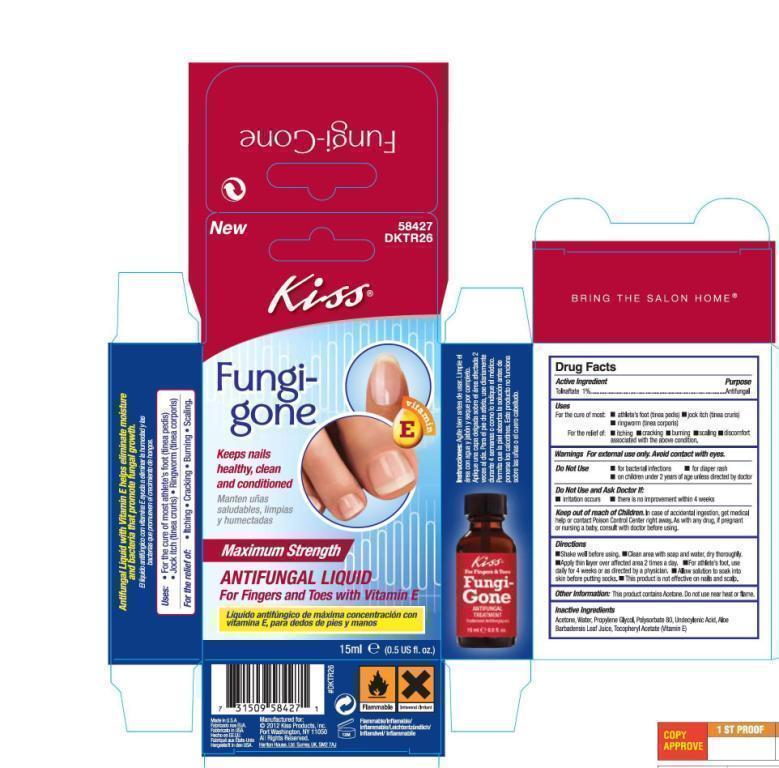 FungiGoneOneOunceLabel.jpg
FungiGoneOneOunceLabel.jpg FungiGoneOneOunceCarton1.jpg
FungiGoneOneOunceCarton1.jpg FungiGoneOneOunceCarton2.jpg
FungiGoneOneOunceCarton2.jpg