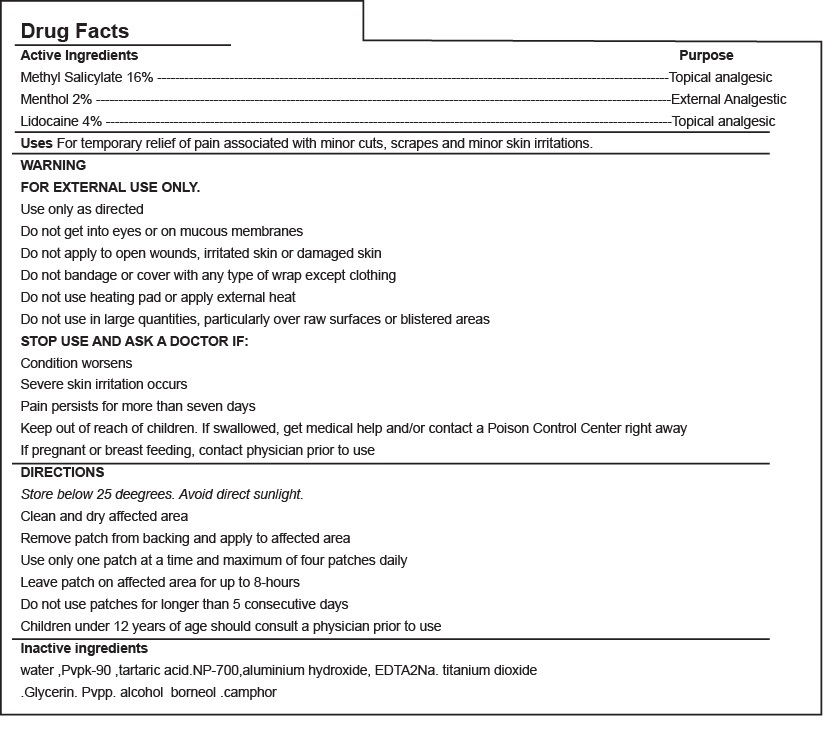
VELMA PAIN RELIEF PATCH- velma pain relief patch patch
Home Aide Diagnostics, Inc.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
VELMA PAIN RELIEF PATCH
| VELMA PAIN RELIEF PATCH
velma pain relief patch patch |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Home Aide Diagnostics, Inc. (783518983) |
Revised: 11/2014
Document Id: 08b1dc5e-16e0-3d48-e054-00144ff8d46c
Set id: 08b1dc5e-16df-3d48-e054-00144ff8d46c
Version: 1
Effective Time: 20141118
Home Aide Diagnostics, Inc.