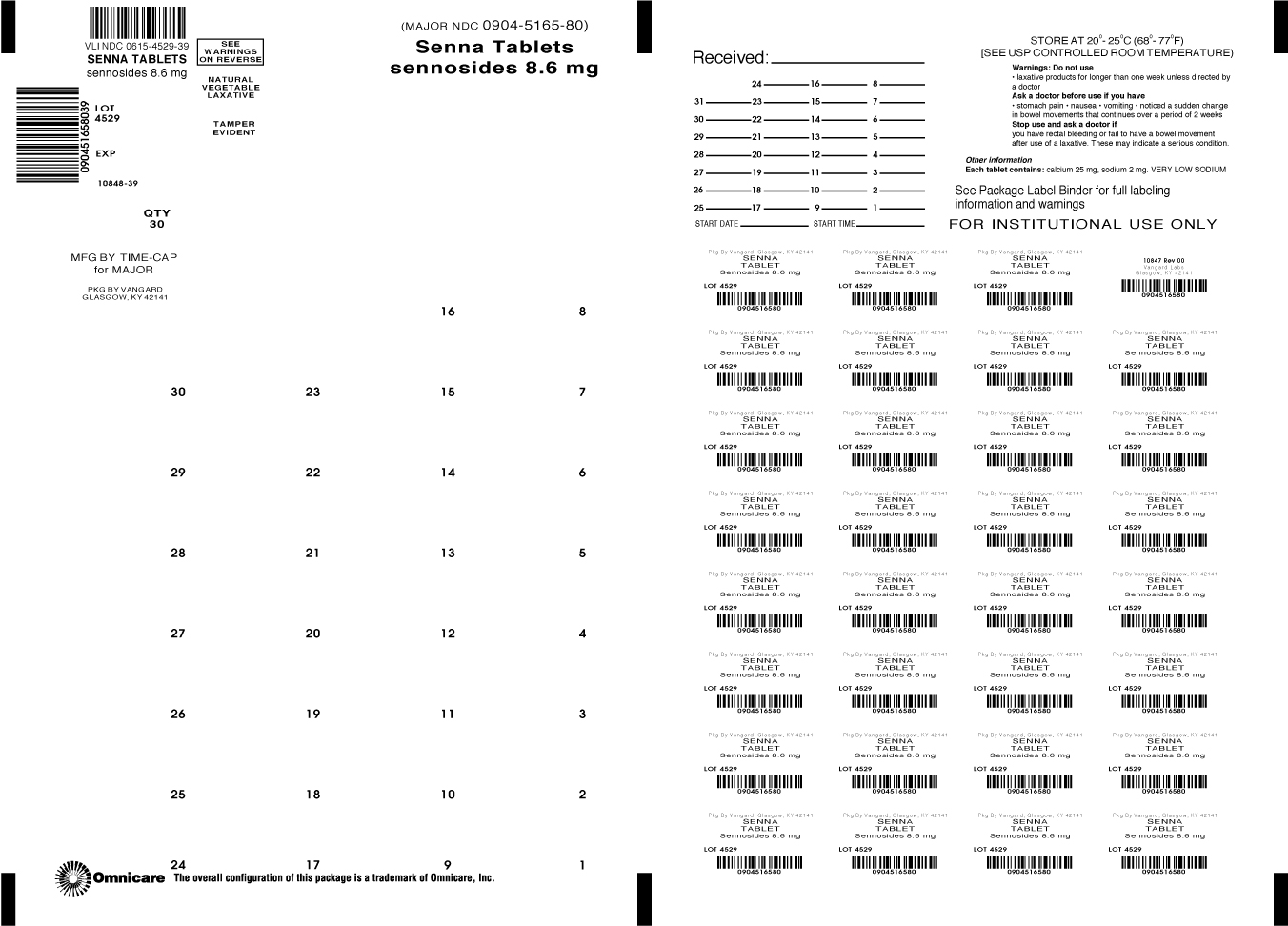
SENNA LAXATIVE- sennosides tablet, coated
NCS HealthCare of KY, LLC dba Vangard Labs
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Senna Tablets
Warnings
Ask a doctor before use if you have
stomach pain, nausea, vomiting, noticed a sudden change in bowel movements that continues over a period of 2 weeks.
Directions
take perferably at bedtime or as directed by a doctor
If you do not have a comforable bowel movement by the second day, increase dose by one tablet (do not exceed maximum dosage) or decrease dose until you are comforable.
| age | starting dosage | maximum dosage | ||||
| adults and children over 12 years | 2 tablets once a day | 4 tablets twice a day | ||||
| children 6 to under 12 years | 1 tablet once a day | 2 tablets twice a day | ||||
| children 2 to under 6 years | 1/2 tablet once a day | 1 tablet twice a day | ||||
| children under 2 years | ask a doctor | ask a doctor | ||||
Other information
Each tablet contains: calcium 25mg, sodium 2mg, VERY LOW SODIUM
store at 25ºC (77ºF) excursions permitted between 15º - 30ºC (59º - 86ºF)
Inactive ingredients
Croscarmellose sodium, dibasic calcium phosphate dihydrate, hypromellose, magnesium stearate, microcrystalline cellulose, mineral oil
| SENNA LAXATIVE
sennosides tablet, coated |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - NCS HealthCare of KY, LLC dba Vangard Labs (050052943) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| NCS HealthCare of KY, LLC dba Vangard Labs | 050052943 | repack(0615-4529) | |