ROHTO ARCTIC- hypromellose, tetrahydrozoline hydrochloride liquid
The Mentholatum Company
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
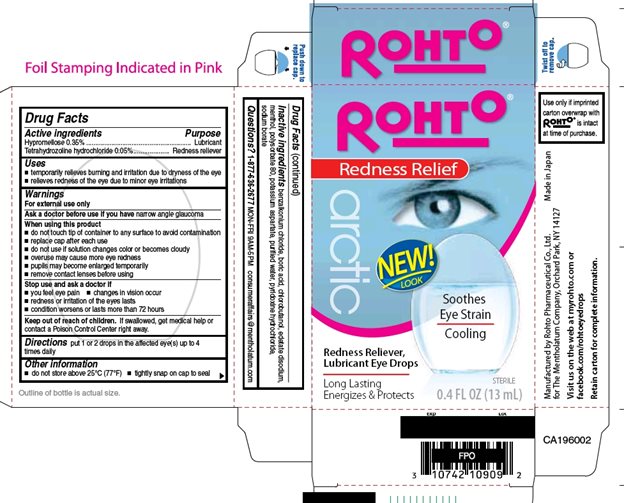
Drug Facts
Uses
- •
- temporarily relieves burning and irritation due to dryness of the eye
- •
- relieves redness of the eye due to minor eye irritations
Warnings
For external use only
Ask a doctor before use if you have narrow angle glaucoma
When using this product
- •
- do not touch tip of container to any surface to avoid contamination
- •
- replace cap after each use
- •
- do not use if solution changes color or becomes cloudy
- •
- overuse may cause more eye redness
- •
- pupils may become enlarged temporarily
- •
- remove contact lenses before using
Directions
put 1 or 2 drops in the affected eye(s) up to 4 times daily
Other information
- •
- do not store above at 25°C (77°F)
- •
- tightly snap on cap to seal
Inactive ingredients
benzalkonium chloride, boric acid, chlorobutanol, edetate disodium, menthol, polysorbate 80, potassium aspartate, purified water, pyridoxine hydrochloride, sodium borate
| ROHTO
ARCTIC
hypromellose, tetrahydrozoline hydrochloride liquid |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - The Mentholatum Company (002105757) |
Revised: 11/2016
Document Id: bf21a686-e306-4dc9-b962-5d313bbc6c9d
Set id: 06b72ff2-2cdb-4a03-8dc1-d2e500c844c1
Version: 4
Effective Time: 20161102
The Mentholatum Company