TRICLONEX T2- triclosan sponge
Nex Medical Antiseptics S.R.L.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
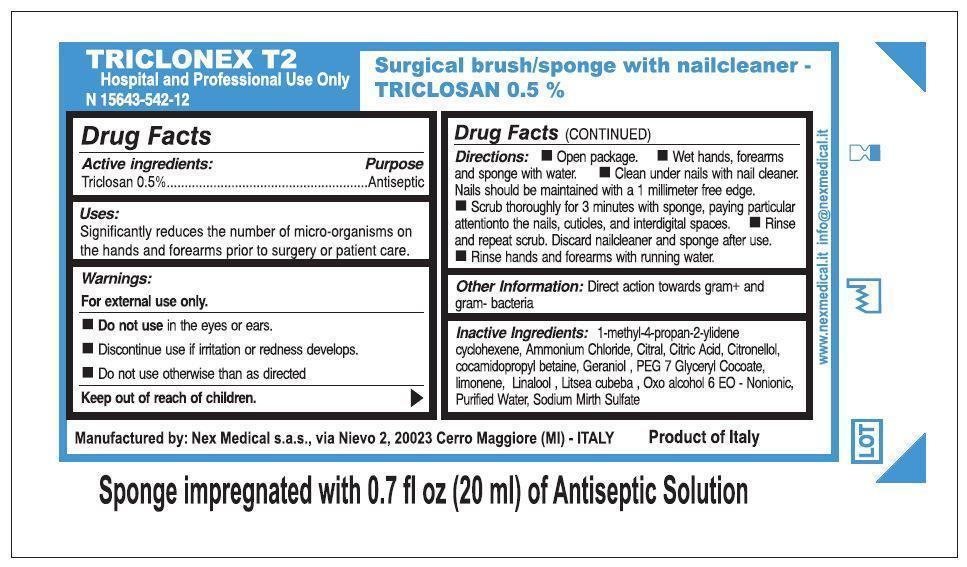
Triclonex T2 Triclosan 0.5%
Uses:
Significantly reduces the number of micro-organisms on
the hands and forearms prior to surgery or patient care.
Warnings:
For external use only.
- Do not use in the eyes or ears.
- Discontinue use if irritation or redness develops.
- Do not use otherwise than as directed
Keep out of reach of children.
Directions:
- Open package.
- Wet hands, forearms and sponge with water.
- Clean under nails with nail cleaner.
Nails should be maintained with a 1 millimeter free edge.
- Scrub thoroughly for 3 minutes with sponge, paying particular
attention to the nails, cuticles, and interdigital spaces.
- Rinse and repeat scrub. Discard nailcleaner and sponge after use.
- Rinse hands and forearms with running water.
Inactive Ingredients:
1-methyl-4-propan-2-yidene cyclohexene,
Ammonium Chloride, Citral, Citric Acid, Citronellol,
cocamidopropyl betaine, Geraniol, PEG 7 Gyceryl Cocoate,
limonene, Linalool, Lisea cubeba, Oxo alcohol 6 EO - Nonionic,
Purified Water, Sodium Mirth Sulfate
TRICLONEX T2
Hospital and Professional Use Only
Surgical brush/sponge with nailcleaner -
TRICLOSAN 0.5%
Manufactured by: Nex Medical s.a.s., via Nievo 2, 20023 Cerro Maggiore (MI) - ITALY
Product of Italy
www.nexmedical.it
info@nexmedical.it
Sponge impregnated with 0.7 fl oz (20 ml) of Antiseptic Solution
| TRICLONEX T2
triclosan sponge |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Nex Medical Antiseptics S.R.L. (434891945) |
| Registrant - Nex Medical Antiseptics S.R.L. (434891945) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Nex Medical Antiseptics S.R.L. | 434891945 | manufacture(50006-300) | |