Label: carbastat- Carbachol injection, solution
-
Contains inactivated NDC Code(s)
NDC Code(s): 58768-735-12 - Packager: Novartis Ophthalmics
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated September 19, 2006
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- N/A - Section Title Not Found In Database
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
CARBASTAT® (Carbachol Intraocular Solution, USP) 0.01% is a sterile balanced salt solution of carbachol for intraocular injection. The active ingredient is represented by the structural formula:
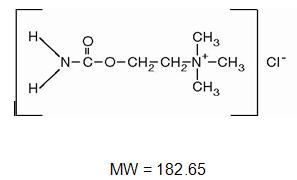
Established name: Carbachol
Chemical name: Ethanaminium, 2-[(aminocarbonyl)oxy]-N,N,N -trimethyl-, chloride.
Each mL contains: Active: Carbachol 0.01%.
Inactive: Sodium chloride 0.64%, potassium chloride 0.075%, calcium chloride dihydrate 0.048%, magnesium chloride hexahydrate 0.03%, sodium acetate trihydrate 0.39%, sodium citrate dihydrate 0.17%, sodium hydroxide and/or hydrochloric acid to adjust pH (5.0-7.5) and water for injection USP.
- CLINICAL PHARMACOLOGY
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
- WARNINGS
-
PRECAUTIONS
Pregnancy: Category C.
There are no adequate and well controlled studies in pregnant women. Carbastat® should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
- ADVERSE REACTIONS
-
DOSAGE AND ADMINISTRATION
Aseptically remove the sterile vial from the blister package by peeling the backing paper and dropping the vial onto a sterile tray. Withdraw the contents into a dry sterile syringe, and replace the needle with an atraumatic cannula prior to intraocular irrigation. No more than one-half milliliter should be gently instilled into the anterior chamber for the production of satisfactory miosis. It may be instilled before or after securing sutures. Miosis is usually maximal within two to five minutes after application.
-
HOW SUPPLIED
CARBASTAT (Carbachol Intraocular Solution, USP) 0.01%
1.5 mL sterile glass vials in cartons of 12 (12 x 1.5 mL)
NDC 58768-735-12
Store at controlled room temperature 15°-30°C (59°-86°F).
Rx only
Manufactured by OMJ Pharmaceuticals, Inc.,
San Germán, P.R. 00683
for Novartis Ophthalmics
Duluth, GA 30097
5007-D
March, 2001
-
INGREDIENTS AND APPEARANCE
CARBASTAT
carbachol injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:58768-735 Route of Administration INTRAOCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Carbachol (UNII: 8Y164V895Y) (Carbachol - UNII:8Y164V895Y) 0.1 mg in 1 mL Inactive Ingredients Ingredient Name Strength calcium chloride dihydrate (UNII: M4I0D6VV5M) 0.481 mg in 1 mL hydrochloric acid (UNII: QTT17582CB) magnesium chloride hexahydrate (UNII: 02F3473H9O) 0.3 mg in 1 mL potassium chloride (UNII: 660YQ98I10) 0.75 mg in 1 mL sodium acetate trihydrate (UNII: 4550K0SC9B) 3.9 mg in 1 mL Sodium chloride (UNII: 451W47IQ8X) 6.4 mg in 1 mL sodium citrate dihydrate (UNII: B22547B95K) 1.7 mg in 1 mL sodium hydroxide (UNII: 55X04QC32I) water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58768-735-12 12 in 1 CARTON 1 1.5 mL in 1 VIAL, GLASS Labeler - Novartis Ophthalmics
