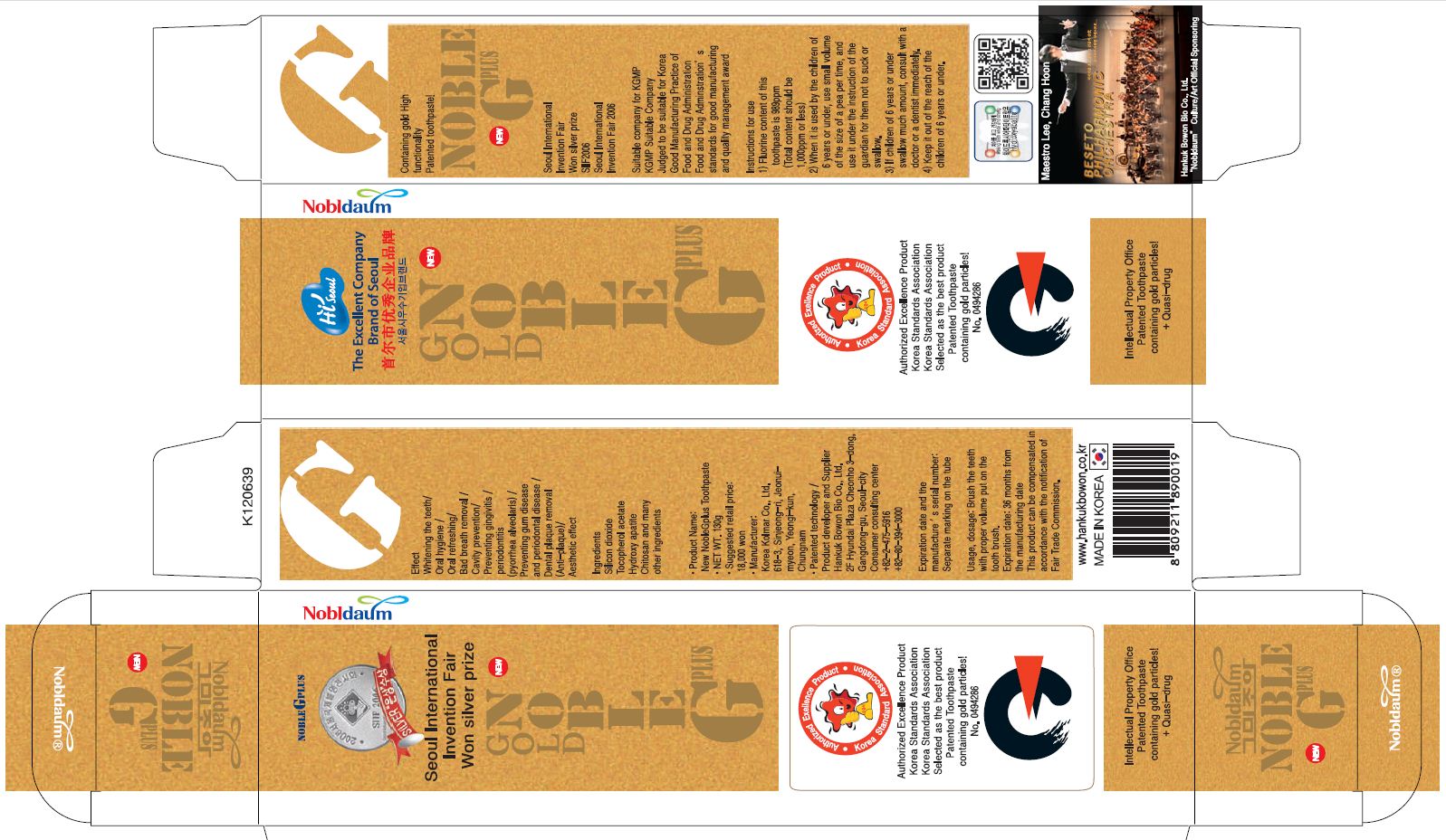
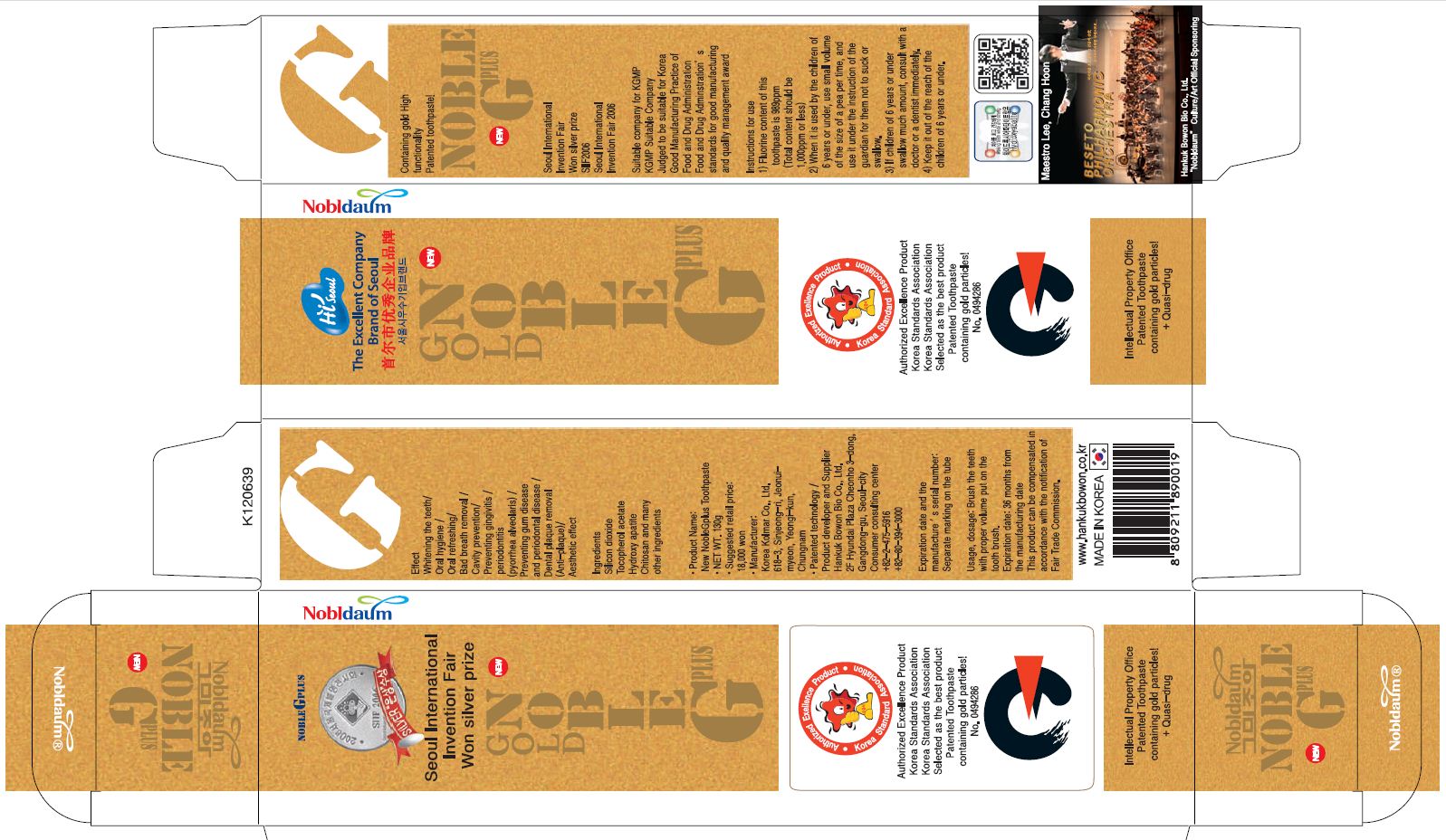
Label: NOBLE G PLUS- xylitol paste, dentifrice
- NDC Code(s): 60319-3001-1
- Packager: Hankuk Bowonbio Co., Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated December 5, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- INACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- WARNINGS
- DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
NOBLE G PLUS
xylitol paste, dentifriceProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:60319-3001 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength XYLITOL (UNII: VCQ006KQ1E) (XYLITOL - UNII:VCQ006KQ1E) XYLITOL 0.7 g in 100 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM MONOFLUOROPHOSPHATE (UNII: C810JCZ56Q) HYDRATED SILICA (UNII: Y6O7T4G8P9) SORBITOL (UNII: 506T60A25R) CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) SODIUM LAURYL SULFATE (UNII: 368GB5141J) SACCHARIN SODIUM ANHYDROUS (UNII: I4807BK602) GOLD (UNII: 79Y1949PYO) GREEN TEA LEAF (UNII: W2ZU1RY8B0) POLYETHYLENE GLYCOL 1500 (UNII: 1212Z7S33A) LEVOMENTHOL (UNII: BZ1R15MTK7) TRIBASIC CALCIUM PHOSPHATE (UNII: 91D9GV0Z28) CHITOSAN OLIGOSACCHARIDE (UNII: 23R93M6Y64) ISOSTEARIC ACID (UNII: X33R8U0062) POLYSORBATE 80 (UNII: 6OZP39ZG8H) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:60319-3001-1 130 g in 1 TUBE; Type 0: Not a Combination Product 12/06/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 11/30/2012 Labeler - Hankuk Bowonbio Co., Ltd (690045133) Registrant - Hankuk Bowonbio Co., Ltd (690045133) Establishment Name Address ID/FEI Business Operations Hankuk Bowonbio Co., Ltd 690045133 manufacture(60319-3001)