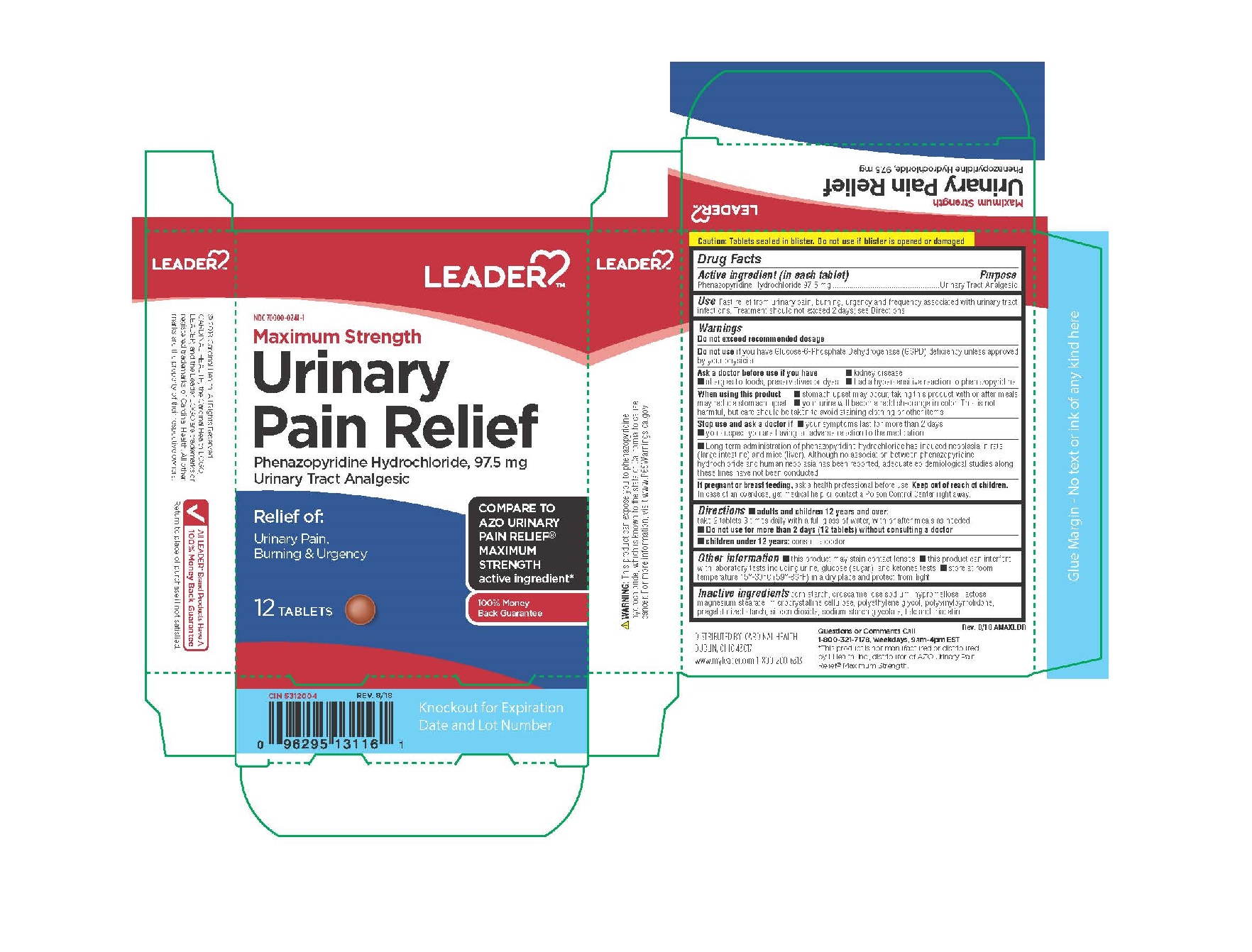
LEADER MAXIMUM STRENGHT URINARY PAIN RELIEF- phenazopyridine hydrochloride tablet
Cardinal Health
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
DRUG FACTS
ASK DOCTOR
ASK DOCTOR BEFORE USE
IF YOU HAVE KIDNEY DISEASE
ALLERGIES TO FOODS,PRESERVATIVES OR DYES
HAD A HYPERSENSITIVE REACTION TO PHENAZOPYRIDINE
WHEN USING
WHEN USING THIS PRODUCT
STOMACH UPSET MAY OCCUR,TAKING THIS PRODUCT WITH OR AFTER MEALS MAY REDUCE STOMACH UPSET
YOUR URINE WILL BECOME REDDISH ORANGE IN COLOR.THIS IS NOT HARMFUL,BUT CARE SHOULD BE TAKEN TO
AVOID STAINING CLOTHING OR OTHER ITEMS.
KEEP OUT OF REACH OF CHILDREN
IN CASE OF OVERDOSE ,GET MEDICAL HELP OR CONTACT A POISON CONTROL CENTER RIGHT AWAY.
INDICATIONS & USAGE
Use; fast relief from urinary pain,burning,urgency and frequency associated with urinary tract infections.
INACTIVE INGREDIENT
Lactose, magnesium silicate, magnesium stearate, microcrystalline cellulose,
pharmaceutical glaze, and sodium starch glycolate.
| LEADER MAXIMUM STRENGHT URINARY PAIN RELIEF
phenazopyridine hydrochloride tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Cardinal Health (097537435) |
| Registrant - Reese Pharmaceutical Co (004172052) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Reese Pharmaceutical Co | 004172052 | relabel(37205-630) , repack(37205-630) , manufacture(37205-630) | |