ULTRA CONTROL STRAWBERRY- sodium fluoride aerosol, foam
WATER PIK, INC.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
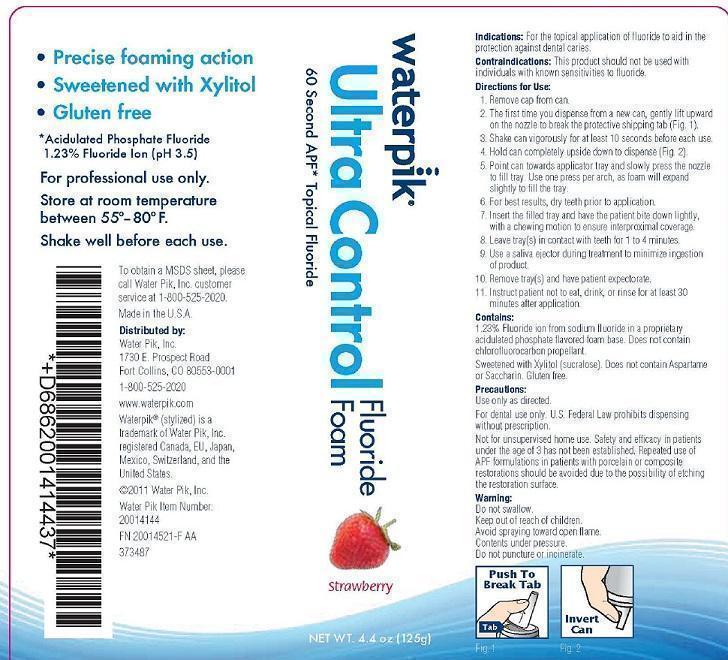
Indications: For the topical application of fluoride to aid in the protection against dental caries.
Contraindications: This product should not be used with individuals with known sensitivities to fluoride.
Directions for Use:
1. Remove cap from can.
2. The first time you dispense from a new can, gently lift upward on the nozzle to break the protective shipping tab (Fig. 1).
3. Shake can vigorously for at least 10 seconds before each use.
4. Hold can completely upside down to dispense (Fig. 2).
5. Point can towards applicator tray and slowly press the nozzle to fill tray. Use one press per arch, as foam will expand slightly to fill the tray.
6. For best results, dry teeth prior to application.
7. Insert the filled tray and have the patient bite down lightly, with a chewing motion to ensure interproximal coverage.
8. Leave tray(s) in contact with teeth for 1 to 4 minutes.
9. Use a saliva ejector during treatment to minimize ingestion of product.
10. Remove tray(s) and have patient expectorate.
11. Instruct patient not to eat, drink, or rinse for at least 30 minutes after application.
Contains:
1.23% Fluoride ion from sodium fluoride in a proprietary acidulated phosphate flavored foam base. Does not contain chlorofluorocarbon propellant.
Sweetened with Xylitol (sucralose). Does not contain Aspartame or Saccharin. Gluten free.
Precautions:
Use only as directed.
For dental use only. U.S. Federal Law prohibits dispensing without prescription.
Not for unsupervised home use. Safety and efficacy in patients under the age of 3 has not been established. Repeated use of APF formulations in patients with porcelain or composite restorations should be avoided due to the possibility of etching the restoration surface.
| ULTRA CONTROL
STRAWBERRY
sodium fluoride aerosol, foam |
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
| Labeler - WATER PIK, INC. (001804074) |
| Registrant - WATER PIK, INC. (001804074) |