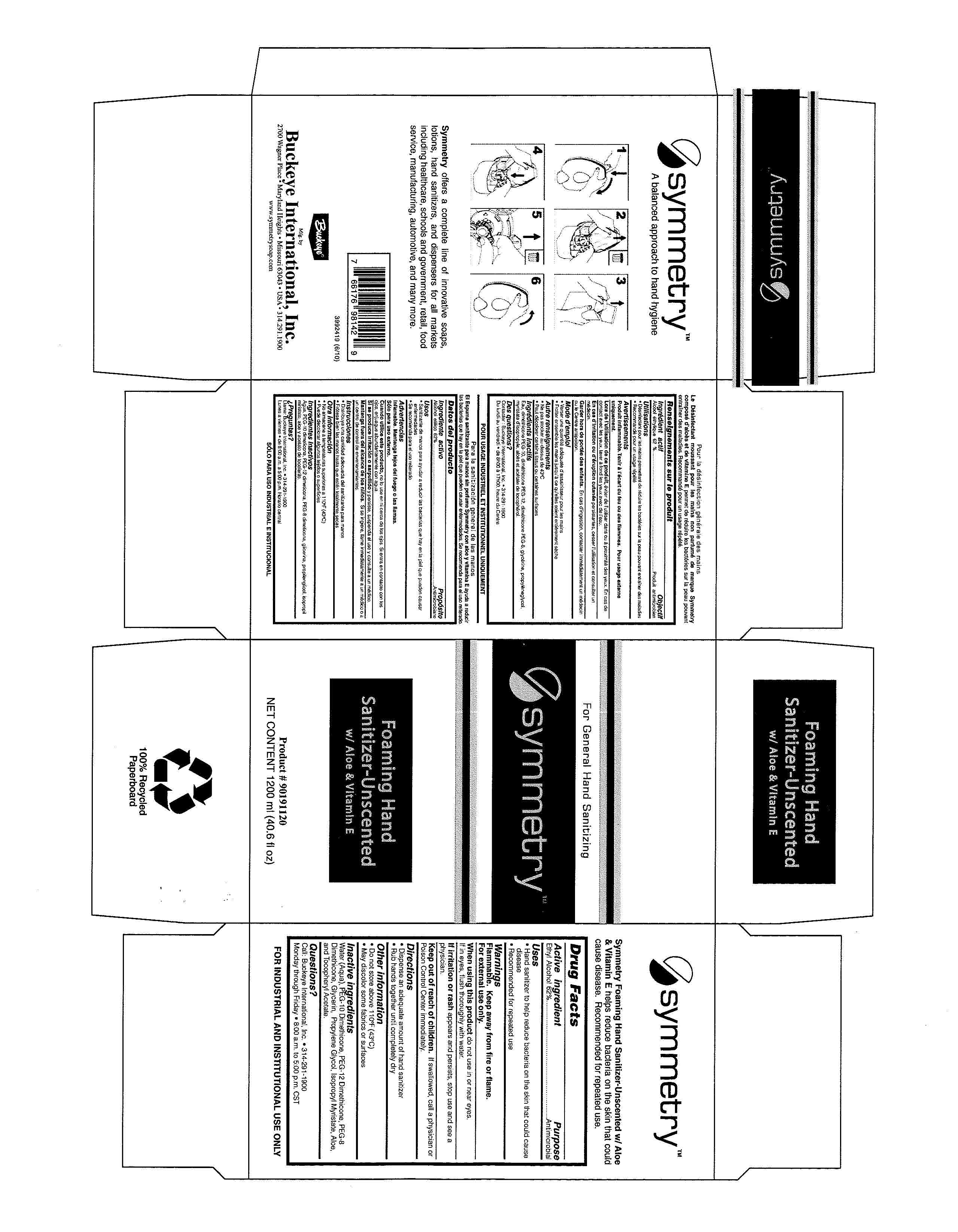
SYMMETRY FOAMING HAND SANITIZER-UNSCENTED- ethyl alcohol liquid
Buckeye International, Inc.
----------
Drug Facts 30805-008
Active ingredient Purpose
Ethyl Alcohol 62%.............................................Antimicrobial
Uses
Hand sanitizer to help reduce bacteria on the skin that could cause disease
Recommended for repeated use
Inactive ingredients
Water (Aqua), PEG-10 Dimethicone, PEG-12 Dimethicone, PEG-8 Dimethicone, Glycerin, Propylene Glycol, Isopropyl Myristate, Aloe, and Tocopheryl Acetate.
| SYMMETRY FOAMING HAND SANITIZER-UNSCENTED
ethyl alcohol liquid |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Buckeye International, Inc. (077132280) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Buckeye International, Inc. | 077132280 | manufacture(30805-008) | |
Revised: 2/2024
Document Id: 10f609af-bb16-9a71-e063-6394a90aae33
Set id: 0194dc3e-5293-415d-a6fb-1da7990cabcf
Version: 2
Effective Time: 20240209
Buckeye International, Inc.