ANTACID- aluminum hydroxide, magnesium hydroxide, simethicone liquid
Western Family Foods Inc
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
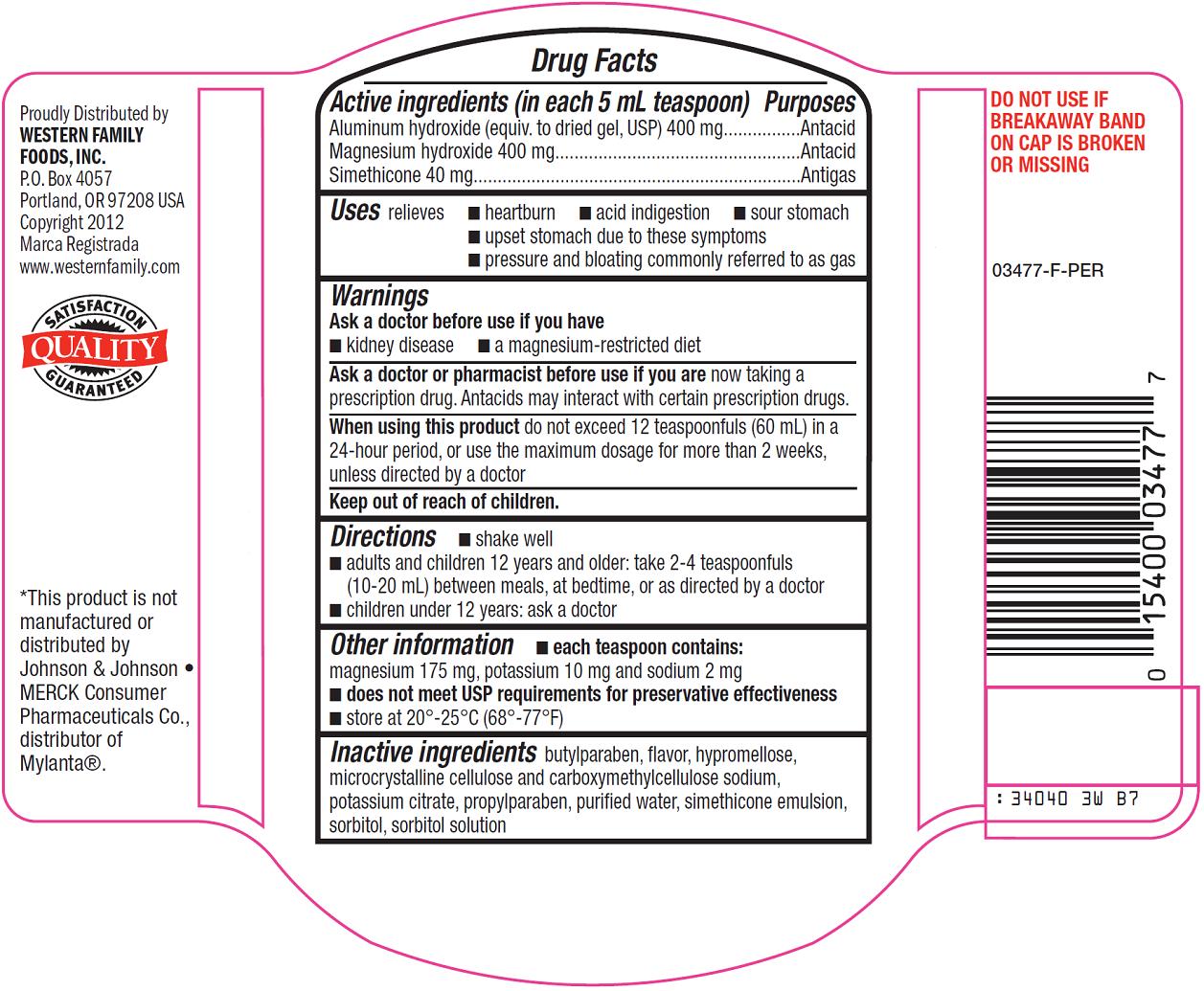
Western Family Antacid Drug Facts
Active ingredient (in each 5 mL teaspoon)
Aluminum hydroxide (equiv. to dried gel, USP) 400 mg
Magnesium hydroxide 400 mg
Simethicone 40 mg
Uses
relieves
- •
- heartburn
- •
- acid indigestion
- •
- sour stomach
- •
- upset stomach due to these symptoms
- •
- pressure and bloating commonly referred to as gas
Warnings
Ask a doctor or pharmacist before use if you are
now taking a prescription drug. Antacids may interact with certain prescription drugs.
Directions
- •
- shake well
- •
- adults and children 12 years and older: take 2-4 teaspoonfuls (10-20 mL) between meals, at bedtime, or as directed by a doctor
- •
- children under 12 years: ask a doctor
Other information
- •
- each teaspoon contains: magnesium 175 mg, potassium 10 mg and sodium 2 mg
- •
- doesnot meet USP requirements for preservative effectiveness
- •
- store at 20º-25ºC (68º-77ºF)
| ANTACID
aluminum hydroxide, magnesium hydroxide, simethicone liquid |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Western Family Foods Inc (192166072) |
Revised: 11/2017
Document Id: 36f5db0c-661a-4828-9b95-6b68405b7dc2
Set id: 013bd74a-3420-4cd4-8546-83d11cae7739
Version: 2
Effective Time: 20171127
Western Family Foods Inc