Label: CYCLOSERINE capsule
- NDC Code(s): 13845-1202-2
- Packager: Parsolex Gmp Center, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 19, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
D -Cycloserine, (R)-4-amino-3-isoxazolidinone,
is a broad-spectrum antibiotic that is produced
by a strain of Streptomyces orchidaceus and has
also been synthesized. Cycloserine is a white
to off-white powder that is soluble in water and
stable in alkaline solution. It is rapidly destroyed
at a neutral or acid pH.
Cycloserine has a pH between 5.5 and 6.5 in a
solution containing 100 mg/mL. The molecular
weight of cycloserine is 102.09, and it has an
empirical formula of C 3H6N2O 2 . The structural
formula of cycloserine is as follows: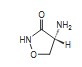
Each capsule contains cycloserine, 250 mg
(2.45 mmol); D & C Yellow No. 10, F D & C Blue
No. 1, F D & C Red No. 3, F D & C Yellow No.
6, gelatin, iron oxide, talc, and titanium dioxide. -
CLINICAL PHARMACOLOGY
After oral administration, cycloserine is
readily absorbed from the gastrointestinal
tract, with peak blood levels occurring in 4
to 8 hours. Blood levels of 25 to 30 μg/mL
can generally be maintained with the usual
dosage of 250 mg twice a day, although the
relationship of plasma levels to dosage is
not always consistent. Concentrations in thecerebrospinal fluid, pleural fluid, fetal blood,
and mother’s milk approach those found in
the serum. Detectable amounts are foundin ascitic fluid, file, sputum, amniotic fluid,
and lung and lymph tissues. Approximately
65% of a single dose of cycloserine can
be recovered in the urine within 72 hours
after oral administration. The remaining
35% is apparently metabolized to unknown
substances. The maximum excretion rate
occurs 2 to 6 hours after administration, with
50% of the drug eliminated in 12 hours.
Mechanism of Action: The antibacterial activity
of Cycloserine results from inhibition of cell-wall
synthesis in susceptible strains of gram-positive
and gram-negative bacteria.
Antibacterial Activity: Cycloserine has been
shown to be active against most isolates of the
following microorganism, both in vitro and in
clinical infections [see Indications and Usage]:
Mycobacterium tuberculosis. -
INDICATIONS AND USAGE
Cycloserine is indicated in the treatment
of active pulmonary and extrapulmonary
tuberculosis (including renal disease) when
the causative organisms are susceptible to
this drug and when treatment with the primary
medications (streptomycin, isoniazid, rifampin,
and ethambutol) has proved inadequate. Like
all antituberculosis drugs, cycloserine should be
administered in conjunction with other effective
chemotherapy and not as the sole therapeutic
agent.
Cycloserine may be effective in the treatment
of acute urinary tract infections caused by
susceptible strains of gram-positive and gram-
negative bacteria. Use of cycloserine in these
infections should be considered only when
more conventional therapy has failed and when
the organism has been demonstrated to be
susceptible to the drug - CONTRAINDICATIONS
-
WARNINGS
Administration of cycloserine should be
discontinued or the dosage reduced if
the patient develops allergic dermatitis or
symptoms of CNS toxicity, such as convulsions,
psychosis, somnolence, depression, confusion,hyperreflexia, headache, tremor, vertigo
paresis, or dysarthria.
The toxicity of cycloserine is closely related to
excessive blood levels (above 30 μg/mL), as
determined by high dosage or inadequate renal
clearance. The ratio of toxic dose to effective
dose in tuberculosis is small.
The risk of convulsions is increased in chronic
alcoholics.
Patients should be monitored by hematologic,
renal excretion, blood level, and liver function
studies. -
PRECAUTIONS
General: Before treatment with cycloserine
is initiated, cultures should be taken and the
organism’s susceptibility to the drug should
be established. In tuberculous infections,
the organism’s susceptibility to the other
antituberculosis agents in the regimen should
also be demonstrated.Anticonvulsant drugs or sedatives may be
effective in controlling symptoms of CNS
toxicity, such as convulsions, anxiety, and
tremor. Patients receiving more than 500 mg
of cycloserine daily should be closely observed
for such symptoms. The value of pyridoxine in
preventing CNS toxicity from cycloserine has
not been proved.
Administration of cycloserine and other
antituberculosis drugs has been associated in
a few instances with vitamin B 12 and/or folic-acid deficiency, megaloblastic anemia, and
sideroblastic anemia. If evidence of anemia
develops during treatment, appropriate studies
and therapy should be instituted.Laboratory Tests: Blood levels should be
determined at least weekly for patients with
reduced renal function, for individuals receiving
a daily dosage of more than 500 mg, and for
those showing signs and symptoms suggestive
of toxicity. The dosage should be adjusted to
keep the blood level below 30 μg/mL.Drug Interactions: Concurrent administration
of ethionamide has been reported to potentiate
neurotoxic side effects.
Alcohol and cycloserine are incompatible,
especially during a regimen calling for large
doses of the latter. Alcohol increases the
possibility and risk of epileptic episodes.
Concurrent administration of isoniazid may
result in increased incidence of CNS effects,
such as dizziness or drowsiness. Dosage
adjustments may be necessary and patients
should be monitored closely for signs of CNS
toxicity.
Carcinogenesis, Mutagenicity, and
Impairment of Fertility: Studies have not
been performed to determine potential for
carcinogenicity. The Ames test and unscheduled
DNA repair test were negative. A study in 2
generations of rats showed no impairment offertility relative to controls for the first mating but
somewhat lower fertility in the second mating.
Pregnancy Category C: There are no
adequate and well-controlled studies with
the use of Cycloserine in pregnant women.
A study in 2 generations of rats given doses
up to 100 mg/kg/day (approximately equivalent
to the maximum recommended human dose
on a body surface area basis) demonstrated
no teratogenic effect in offspring. Cycloserine
should be used during pregnancy only if thepotential benefit justifies the potential risk to the
fetus.Nursing Mothers: Because of the potential
for serious adverse reactions in nursing infants
from cycloserine, a decision should be made
whether to discontinue nursing or to discontinue
the drug, taking into account the importance of
the drug to the mother.
Usage in Pediatric Patients: Safety and
effectiveness in pediatric patients have not been
established.
Geriatric Use: Clinical studies of cycloserinedid not include sufficient numbers of subjects
aged 65 and over to determine whether they
responded differently from younger subjects.
Other reported clinical experience has notidentified differences in responses between
the elderly and younger patients. In general,
dose selection for an elderly patient should be
cautious, usually starting at the low end of thedosing range, reflecting the greater frequency
of decreased hepatic, renal, or cardiac function,
and of concomitant disease or other drug
therapy.
This drug is known to be substantially excreted
by the kidney, and the risk of toxic reactions
to this drug may be greater in patients with
impaired renal function. Because elderly
patients are more likely to have decreased renal
function, care should be taken in dose selection,
and it may be useful to monitor renal function.
The toxicity of cycloserine is closely related
to excessive blood levels (above 30 μg/mL)
as determined by high dosage or inadequate
renal clearance (see WARNINGS). Blood
levels should be determined at least weekly
for patients with reduced renal function, for
individuals receiving a daily dosage of more
than 500 mg, and for those showing signs
and symptoms suggestive of toxicity. The
dosage should be adjusted to keep the blood
level below 30 μg/mL (see PRECAUTIONS,Laboratory Tests).
-
ADVERSE REACTIONS
Most adverse reactions occurring during therapy
with cycloserine involve the nervous system or
are manifestations of drug hypersensitivity. The
following side effects have been observed in
patients receiving cycloserine:
Nervous system symptoms (which appear to
be related to higher dosages of the drug, i.e.,
more than 500 mg daily)
• Convulsions
• Drowsiness and somnolence
• Headache
• Tremor
• Dysarthria
• Vertigo• Confusion and disorientation with loss of
memory
• Psychoses, possibly with suicidal tendencies
• Character changes
• Hyperirritability
• Aggression
• Paresis
• Hyperreflexia
• Paresthesia
• Major & minor (localized) clonic seizures
• ComaCardiovascular: Sudden development of
congestive heart failure in patients receiving
1 to 1.5 g of cycloserine daily has been
reported.
Allergy (apparently not related to dosage)
Skin rash
Miscellaneous: Elevated serum transaminase,
especially in patients with preexisting liver
disease -
OVERDOSAGE
Signs and Symptoms: Acute toxicity from
cycloserine can occur if more than 1 g is
ingested by an adult. Chronic toxicity from
cycloserine is dose related and can occur if
more than 500 mg is administered daily. The
central nervous system is the most common
organ system involved with toxicity. Toxic effects
may include headache, vertigo, confusion,
drowsiness, hyperirritability, paresthesias,
dysarthria, psychosis paresis, convulsions, and
coma.
Treatment: In adults, many of the neurotoxic
effects of cycloserine can be both treated and
prevented with the administration of 200 to 300 mg
of pyridoxine daily.
Hemodialysis has been shown to remove
cycloserine from the bloodstream. This
procedure should be reserved for patients with
life threatening toxicity that is unresponsive to
less invasive therapy. -
DOSAGE AND ADMINISTRATION
Cycloserine is effective orally and is currently
administered only by this route. The usual
dosage is 500 mg to 1 g daily in divided doses
monitored by blood levels. 2 The initial adult
dosage most frequently given is 250 mg twicedaily at 12-hour intervals for the first 2 weeks.
A daily dosage of 1 g should not be exceeded. -
HOW SUPPLIED
Cycloserine is available as a 250 mg capsule
with an opaque red cap and opaque gray body
imprinted with “PGC” and “F04” in edible black
ink on both the cap and the body.Aluminum blisters (a pack of 3 cards each with
10 capsules). NDC 13845-1202-2.
Store at controlled room temperature,
20° to 25°C (68° to 77°F) [see USP Controlled
Room Temperature]. - REFERENCES
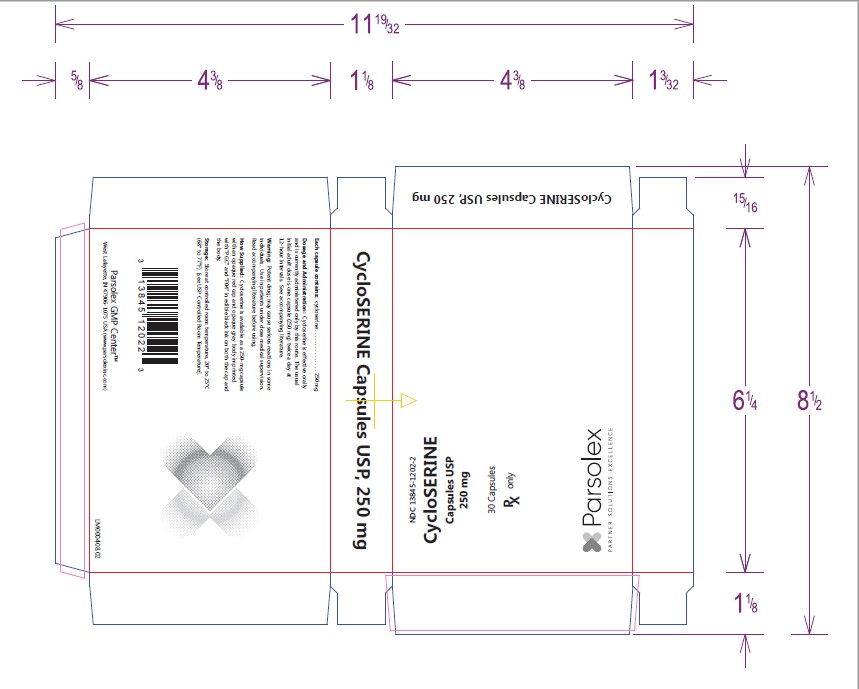
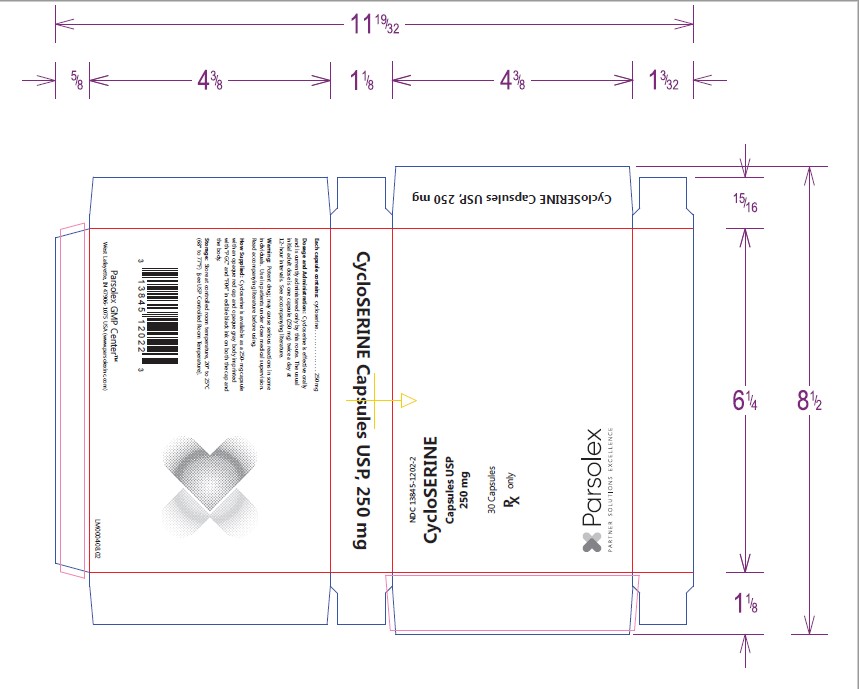
- PACKAGE LABEL DISPLAY
-
INGREDIENTS AND APPEARANCE
CYCLOSERINE
cycloserine capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:13845-1202 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CYCLOSERINE (UNII: 95IK5KI84Z) (CYCLOSERINE - UNII:95IK5KI84Z) CYCLOSERINE 250 mg Product Characteristics Color red (Cap: BOQ - Op. Red 353) , gray (Body: AWZ - Op. Grey 284) Score no score Shape CAPSULE (Imprint on both cap and body) Size 20mm Flavor Imprint Code PGC;F04 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13845-1202-2 3 in 1 CARTON 09/12/2013 1 10 in 1 BLISTER PACK; Type 0: Not a Combination Product 
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA060593 09/12/2013 Labeler - Parsolex Gmp Center, Inc. (159802532) Registrant - Parsolex Gmp Center, Inc. (159802532) Establishment Name Address ID/FEI Business Operations Parsolex Gmp Center, Inc. 159802532 manufacture(13845-1202)