ALLERGY ANTIHISTAMINE- diphenhydramine hcl tablet
EQUALINE (SuperValu)
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
DRUG FACTS
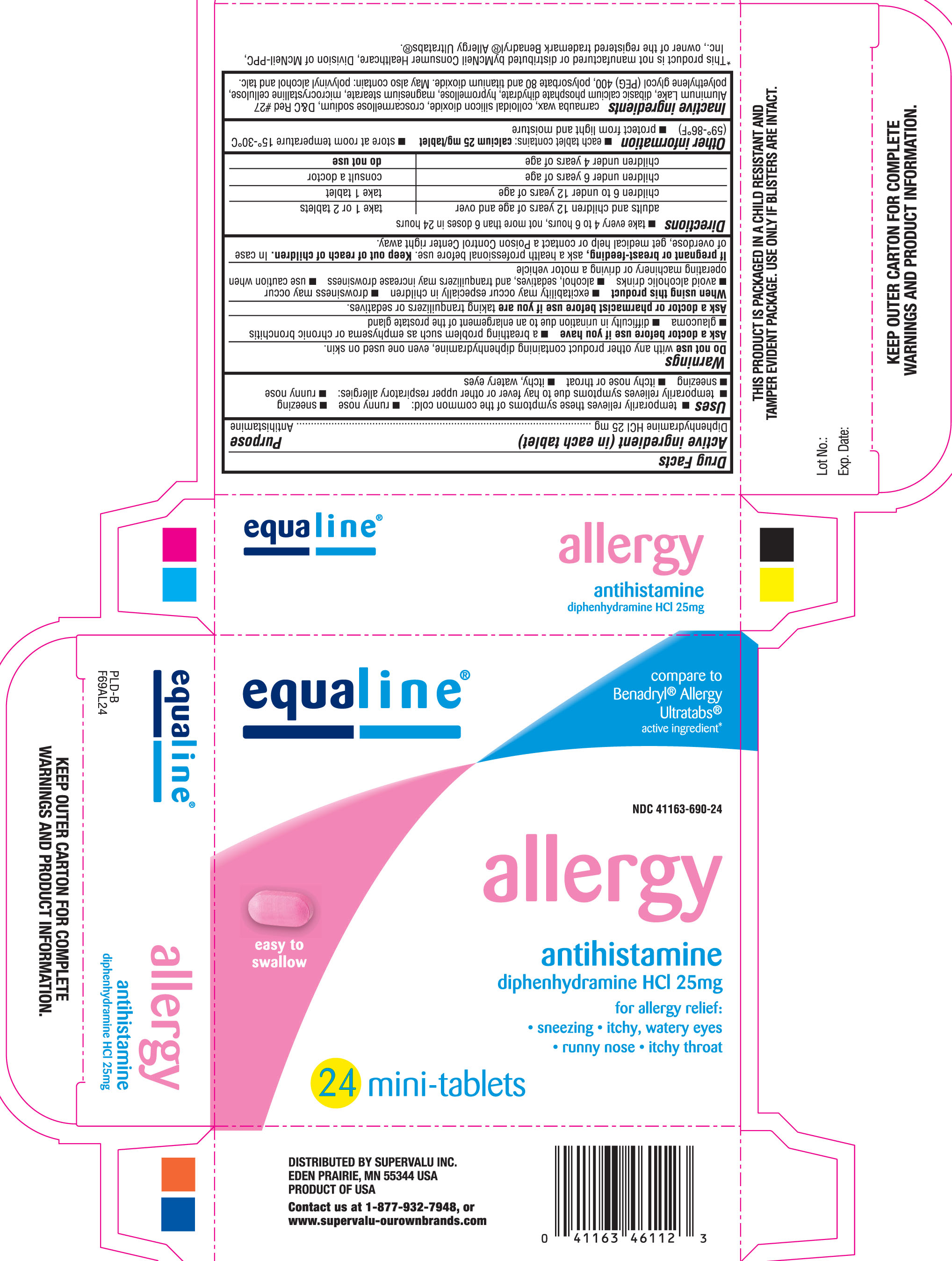
Uses
temporarily relieves these symptoms of the common cold:
- runny nose
- sneezing
temporarily relieves symptoms due to hay fever or other upper respiratory allergies:
- runny nose
- sneezing
- itching nose or throat
- itchy, watery eyes
Warnings
Ask a doctor before use if you have
-
a breathing problem such as emphysema or chronic bronchitis
-
glaucoma
-
difficulty in urination due to an enlargement of the prostate gland
Directions
- take every 4 to 6 hours, not more than 6 doses in 24 hours
| adults and children 12 years of age and over | take 1 or 2 tablets |
| children 6 to under 12 years of age | take 1 tablet |
| children under 6 years of age | consult a doctor |
| children under 4 years of age | do not use |
Other information
- each tablet contains: calcium 25 mg/ tablet
- store at room temperature 15°-30°C (59°-86°F)
- protect from light and moisture
Inactive ingredients
carnauba wax, colloidal silicon dioxide, croscarmellose sodium, D&C Red #27 Aluminum Lake, dibasic calcium phosphate dihydrate, hypromellose, magnesium stearate, microcrystalline cellulose, polyethylene glycol (PEG) 400, polysorbate 80 and titanium dioxide
May also contain: polyvinyl alcohol and talc.
Principal Display Panel
Compare to Benadryl® Allergy ultratabs® Active Ingredient*
Allergy
Antihistamine
For allergy relief:
sneezing, itchy & watery eyes, runny nose & itchy throat
Diphenhydramine HCl 25 mg
*This product is not manufactured or distributed by McNeil Consumer Healthcare, Division of McNeil-PPC, Inc., owner of the registered trademark Benadryl® Allergy Ultratabs®
KEEP OUTER CARTON FOR COMPLETE WARNINGS AND PRODUCT INFORMATION
THIS PRODUCT IS PACKAGED IN A CHILD RESISTANT AND TAMPER EVIDENT PACKAGE. USE ONLY IF BLISTERS ARE INTACT.
DISTRIBUTED BY SUPERVALU INC.
EDEN PRAIRIE, MN 55344 USA
PRODUCT OF USA
CONTACT US AT 1-877-932-7948, OR WWW.SUPERVALU-OUROWNBRANDS.COM
| ALLERGY
ANTIHISTAMINE
diphenhydramine hcl tablet |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Labeler - EQUALINE (SuperValu) (006961411) |