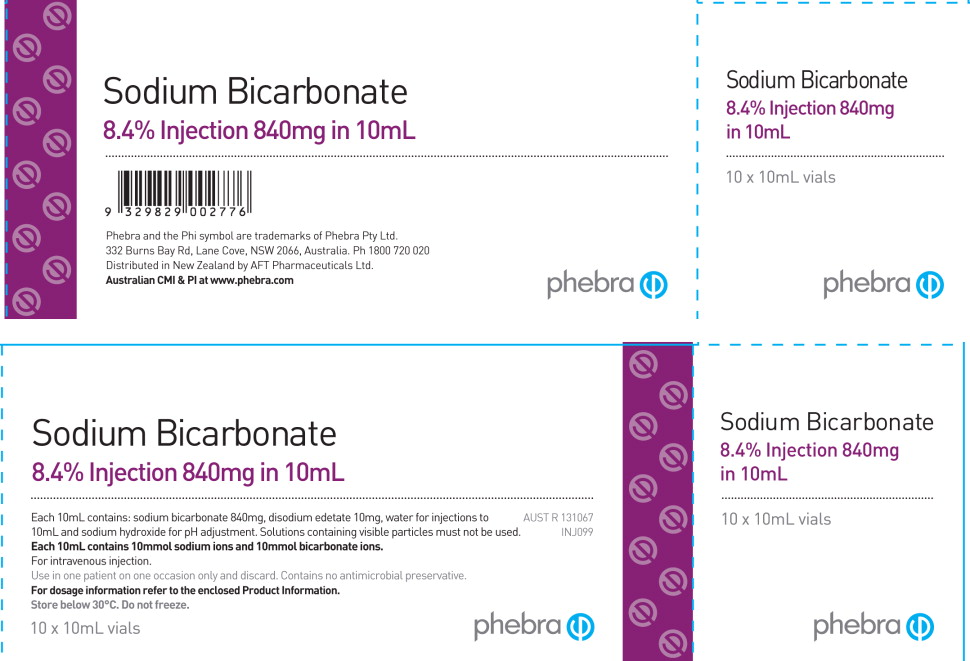
SODIUM BICARBONATE- sodium bicarbonate solution
Sagent Pharmaceuticals
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
SAGENT™
Sagent Pharmaceuticals, Inc.
1901 N. Roselle Road, Suite 700
Schaumburg, IL 60195
(847) 908-1600 Main
(847) 908-1601 Fax
www.SagentPharma.com
URGENT – Sodium Bicarbonate Injection Shortage Update
January 7, 2013
Dear Healthcare Professional,
Due to the current critical shortage of Sodium Bicarbonate Injection, USP in the United States (US) market, Sagent Pharmaceuticals, Inc. (Sagent) is coordinating with the Food and Drug Administration (FDA) to increase the availability of Sodium Bicarbonate Injection. In cooperation with the FDA, Sagent has initiated temporary importation of a non FDA approved 8.4% Sodium Bicarbonate Injection (1 mEq/mL) from Phebra Pty Ltd (Phebra), Australia into the US market.
Phebra's Sodium Bicarbonate Injection contains the same active ingredient, Sodium Bicarbonate, in the same strength and concentration, 8.4% (1 mEq/mL) as the US registered Sodium Bicarbonate Injection, USP by Hospira. However, it is important to note that Phebra's Sodium Bicarbonate Injection (1 mEq/mL), is provided only in a Single Use 10 mL vial, whereas Hospira's product is provided in 50 mL single-dose vials and syringes. Any unused portion of Phebra's Sodium Bicarbonate Injection (1 mEq/mL) should be discarded after a single use.
Please refer to the US approved package insert at http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.DrugDetails for the full prescribing information for8.4% Sodium Bicarbonate Injection (1 mEq/mL).
Phebra's Sodium Bicarbonate labeling does not include an NDC number. To order Phebra's 8.4% Sodium Bicarbonate Injection, (1 mEq/mL), 10 mL vial, please contact your Sagent Regional Business Manager or call Sagent's Customer Service at 1-866-625-1618 and reference item number 32982.
To report adverse events or medication errors among patients who have received Phebra's 8.4% Sodium Bicarbonate Injection, (1 mEq/mL), 10 mL vial, please contact Medical Affairs by phone at 1-866-625-1618. Adverse events may also be reported to FDA's MedWatch Adverse Reporting Program either online, by regular mail or fax:
- Online: www.fda.gov/medwatch/report.htm
- Regular Mail: use postage-paid FDA Form 3500 available at www.fda.gov/Medwatch/getforms.htm. Mail to: MedWatch, FDA, 5600 Fishers Lane, Rockville, MD 20852-9787
- Fax: 1-800-FDA-0178
If you have any questions about the information contained in this letter or the safe and effective use of Phebra's 8.4% Sodium Bicarbonate Injection, (1 mEq/mL), 10 mL vial, please contact Sagent's Medical Affairs at 1-866-625-1618.
At this time, no other entity except Sagent Pharmaceuticals, Inc. is authorized by the FDA to import or distribute Phebra's 8.4% Sodium Bicarbonate Injection, (1 mEq/mL), 10 mL vials, in the United States. Any sales of this product from any entity other than Sagent Pharmaceuticals, Inc. or a distributor or re-seller authorized by Sagent Pharmaceuticals, Inc. could be a violation of the Federal Food, Drug and Cosmetic Act and is subject to enforcement by the FDA.
Sincerely,
Thomas J. Moutvic
Vice President, Regulatory Affairs
Sagent Pharmaceuticals, Inc.
| SODIUM BICARBONATE
sodium bicarbonate solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Sagent Pharmaceuticals (796852890) |