Label: MIRALAX- polyethylene glycol 3350 powder, for solution
-
NDC Code(s):
11523-4357-1,
11523-4357-2,
11523-4357-3,
11523-4357-5, view more11523-7234-1, 11523-7234-2, 11523-7234-3, 11523-7234-4, 11523-7234-5, 11523-7234-6, 11523-7234-9, 11523-7268-3, 11523-7268-4, 11523-7268-7, 11523-7268-8, 11523-7268-9, 11523-7341-1, 11523-7341-2
- Packager: Bayer HealthCare LLC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated December 4, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient (in each dose) (Bottle Only)
- Purpose
- Active ingredient (in each dose) (Packet Only)
- Purpose
- Use
-
Warnings
Ask a doctor before use if you have
- nausea, vomiting or abdominal pain
- a sudden change in bowel habits that lasts over 2 weeks
- irritable bowel syndrome
-
Directions (Bottle Only)
- do not take more than directed unless advised by your doctor
- the bottle top is a measuring cap marked to contain 17 grams of powder when filled to the indicated line (white section in cap)
- adults and children 17 years of age and older:
- use once a day
- fill to top of white section in cap which is marked to indicate the correct dose (17 g)
- stir and dissolve in any 4 to 8 ounces of beverage (cold, hot or room temperature) then drink
- do not combine with starch-based thickeners used for difficulty swallowing
- ensure that the powder is fully dissolved before drinking
- do not drink if there are any clumps
- do not use no more than 7 days
- children 16 years of age or under: ask a doctor
-
Directions (Packet Only)
- do not take more than directed unless advised by your doctor
- adults and children 17 years of age and older:
- use once a day
- stir and dissolve one packet of powder (17 g) in any 4 to 8 ounces of beverage (cold, hot or room temperature) then drink
- do not combine with starch-based thickeners used for difficulty swallowing
- ensure that the powder is fully dissolved before drinking
- do not drink if there are any clumps
- do not use more than 7 days
- children 16 years of age or under: ask a doctor
- Other information
- Inactive ingredients
- Questions or comments?
-
PRINCIPAL DISPLAY PANEL - 17 g × 20 Packet Carton
20
Single Doses
#1 DOCTOR RECOMMENDED BRAND
MiraLAX ®
Polyethylene Glycol 3350, Powder for Solution, Osmotic Laxative
Mix-In Pax ®
- Relieves Occasional Constipation/Irregularity
- Softens Stool
20
ONCE-DAILY DOSES
Bayer
Unflavored Powder Dissolves in ANY Beverage!
UNFLAVORED
POWDER
GRIT FREE20 PACKETS
NET WT 0.5 OZ
(17g) EACH

-
PRINCIPAL DISPLAY PANEL - 17 g × 10 Packet Carton
10
Single Doses
#1 DOCTOR RECOMMENDED BRAND
MiraLAX ®
Polyethylene Glycol 3350, Powder for Solution, Osmotic LaxativeMix-In Pax ®
- Relieves Occasional Constipation/Irregularity
- Softens Stool
10
ONCE-DAILY DOSES
Bayer
Unflavored Powder Dissolves in ANY Beverage!
UNFLAVORED
POWDER
GRIT FREE10 PACKETS
NET WT 0.5 OZ(17g) EACH

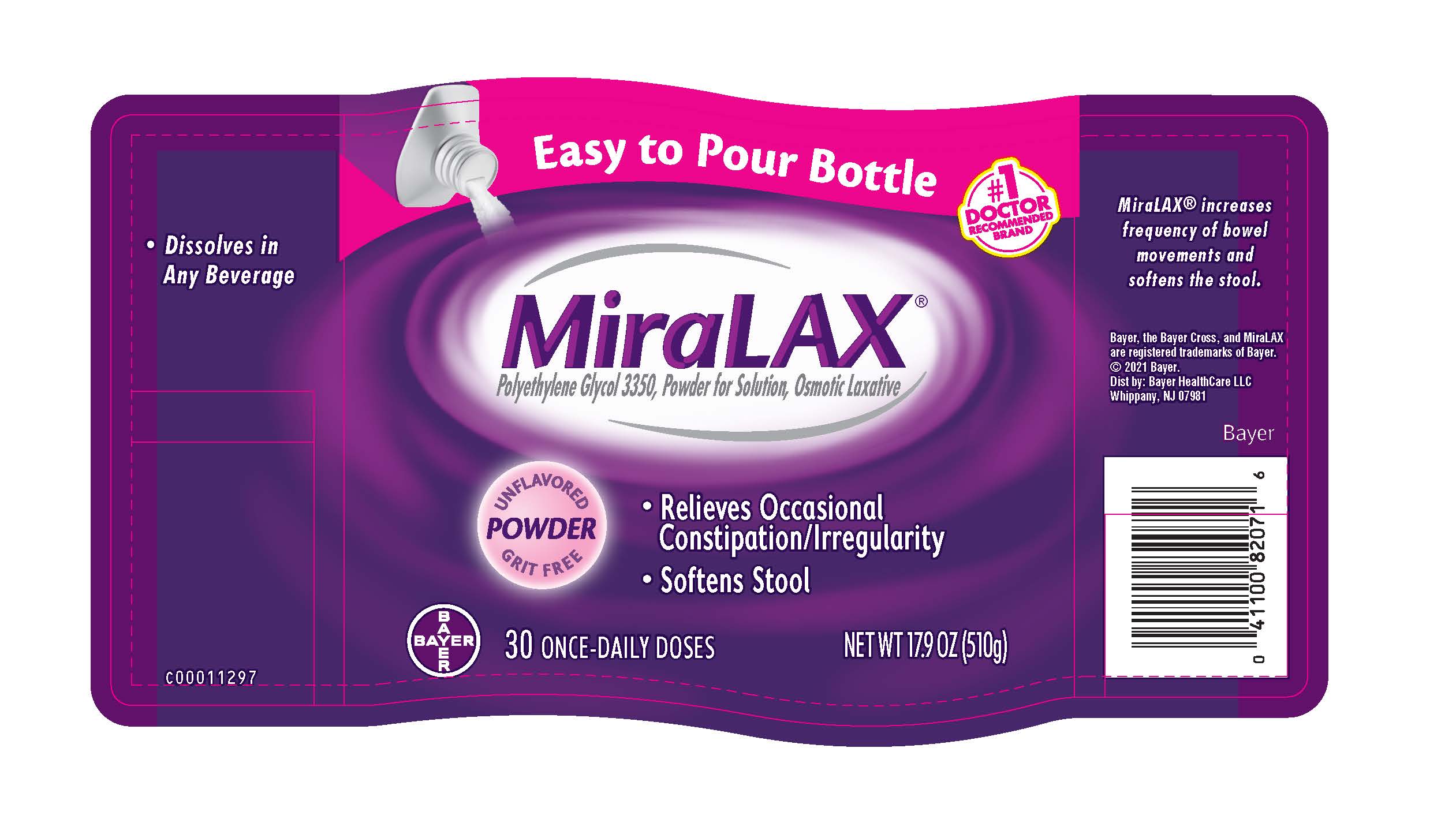
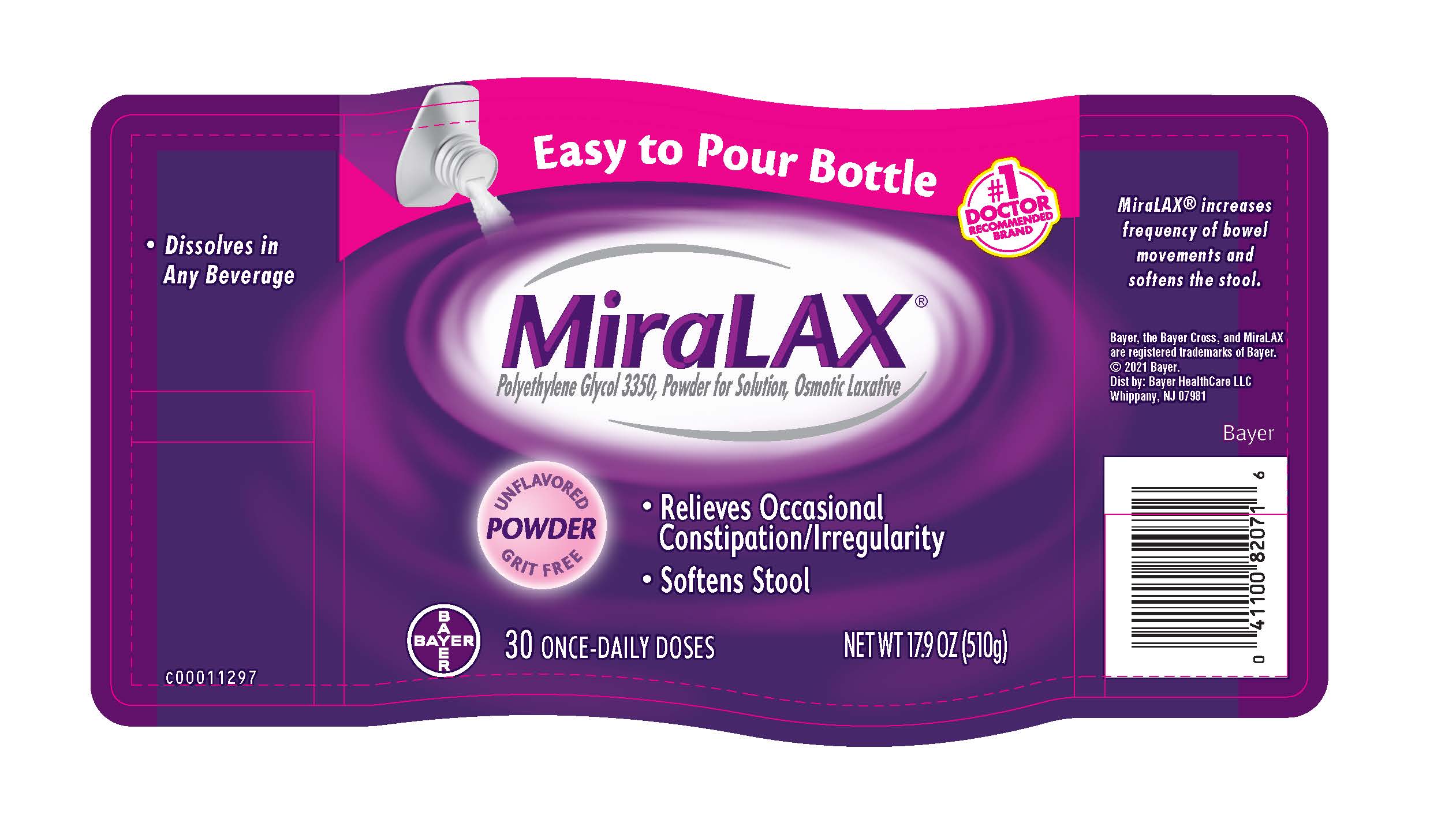
- PRINCIPAL DISPLAY PANEL - 30 Dose (510 g) Bottle Label
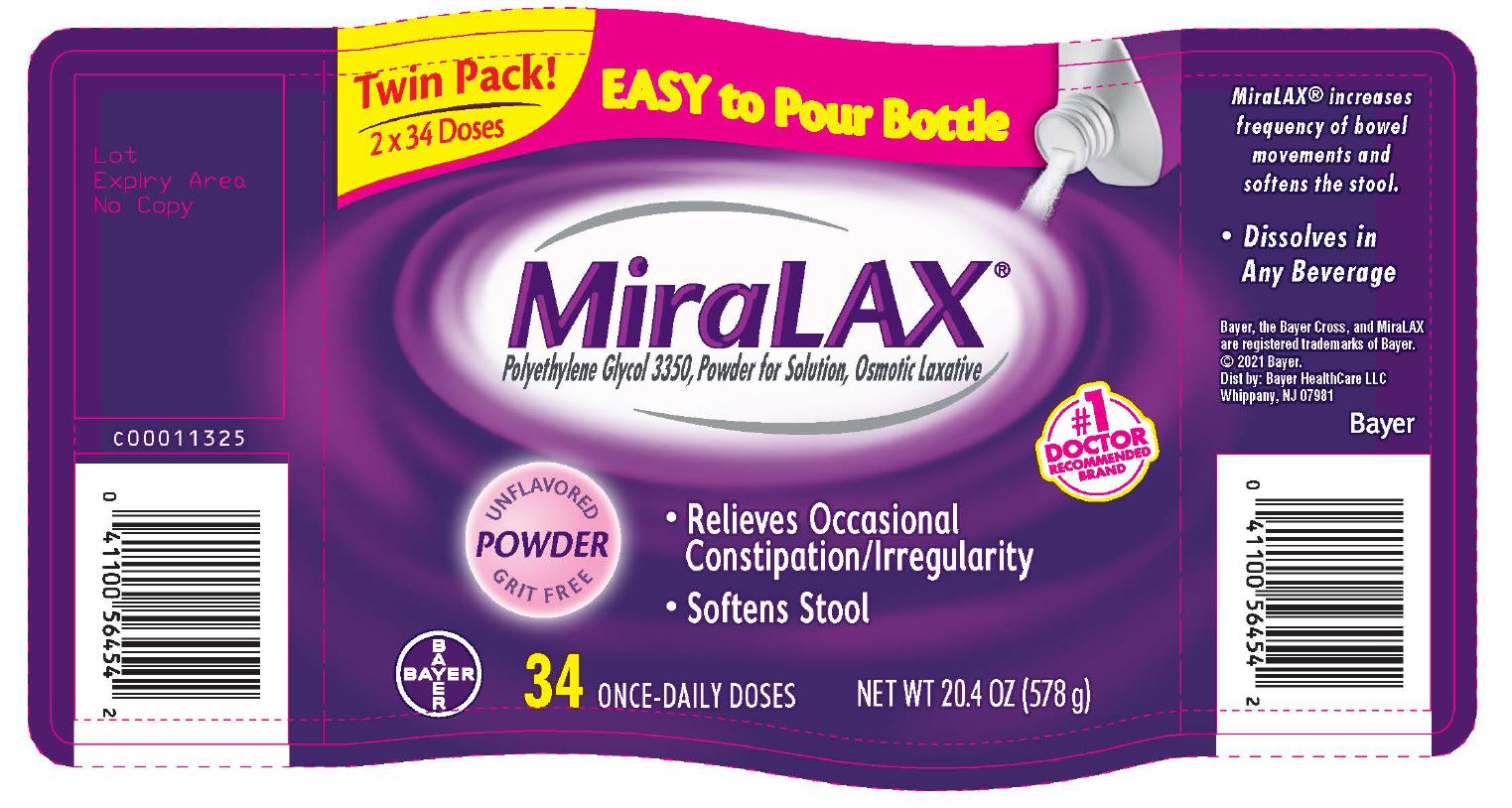
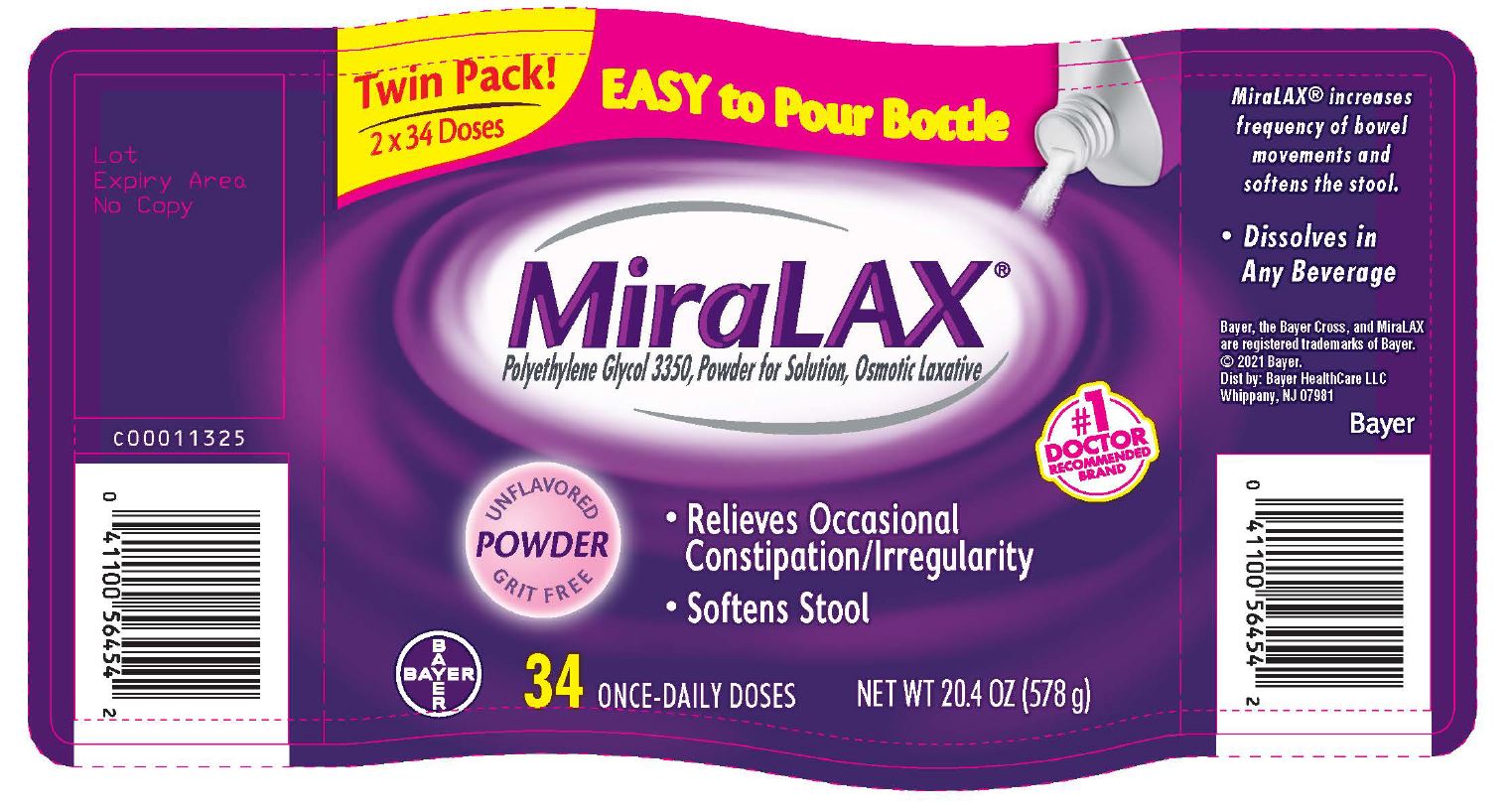
- PRINCIPAL DISPLAY PANEL - Twinpack 2x34 Dose (578 g) Bottle Label
-
INGREDIENTS AND APPEARANCE
MIRALAX
polyethylene glycol 3350 powder, for solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11523-7268 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) (POLYETHYLENE GLYCOL 3350 - UNII:G2M7P15E5P) POLYETHYLENE GLYCOL 3350 17 g in 17 g Product Characteristics Color white (Colorless upon dissolution) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11523-7268-3 10 in 1 CARTON 02/01/2007 1 17 g in 1 PACKET; Type 0: Not a Combination Product 2 NDC:11523-7268-8 24 in 1 CARTON 02/01/2007 2 17 g in 1 PACKET; Type 0: Not a Combination Product 3 NDC:11523-7268-7 5 in 1 CARTON 02/01/2007 01/01/2015 3 17 g in 1 PACKET; Type 0: Not a Combination Product 4 NDC:11523-7268-4 765 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 02/01/2007 5 NDC:11523-7268-9 20 in 1 CARTON 02/01/2007 5 17 g in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022015 02/01/2007 MIRALAX
polyethylene glycol 3350 powder, for solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11523-7234 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) (POLYETHYLENE GLYCOL 3350 - UNII:G2M7P15E5P) POLYETHYLENE GLYCOL 3350 17 g in 17 g Product Characteristics Color white (Colorless upon dissolution) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11523-7234-1 17 g in 1 PACKET; Type 0: Not a Combination Product 02/01/2007 2 NDC:11523-7234-2 119 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 02/01/2007 3 NDC:11523-7234-3 238 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 02/01/2007 4 NDC:11523-7234-4 510 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 02/01/2007 5 NDC:11523-7234-5 12 in 1 CARTON 02/01/2007 01/01/2012 5 17 g in 1 PACKET; Type 0: Not a Combination Product 6 NDC:11523-7234-6 17 g in 1 PACKET; Type 0: Not a Combination Product 02/01/2007 7 NDC:11523-7234-9 2 in 1 CELLO PACK 02/01/2007 01/01/2014 7 510 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022015 02/01/2007 MIRALAX
polyethylene glycol 3350 powder, for solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11523-7341 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) (POLYETHYLENE GLYCOL 3350 - UNII:G2M7P15E5P) POLYETHYLENE GLYCOL 3350 17 g in 17 g Product Characteristics Color white (Colorless upon dissolution) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11523-7341-1 612 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 02/01/2007 2 NDC:11523-7341-2 3 in 1 CARTON 02/01/2007 2 17 g in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022015 02/01/2007 MIRALAX
polyethylene glycol 3350 powder, for solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11523-4357 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) (POLYETHYLENE GLYCOL 3350 - UNII:G2M7P15E5P) POLYETHYLENE GLYCOL 3350 17 g in 17 g Product Characteristics Color white (colorless upon dissolution) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11523-4357-1 2 in 1 PACKAGE, COMBINATION 02/01/2007 01/01/2018 1 765 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 2 NDC:11523-4357-3 2 in 1 PACKAGE, COMBINATION 02/01/2007 2 578 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 3 NDC:11523-4357-5 24 in 1 CARTON 05/01/2018 3 17 g in 1 PACKET; Type 0: Not a Combination Product 4 NDC:11523-4357-2 2 in 1 CARTON 02/01/2007 4 20 in 1 CARTON 4 17 g in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022015 02/01/2007 Labeler - Bayer HealthCare LLC. (112117283)