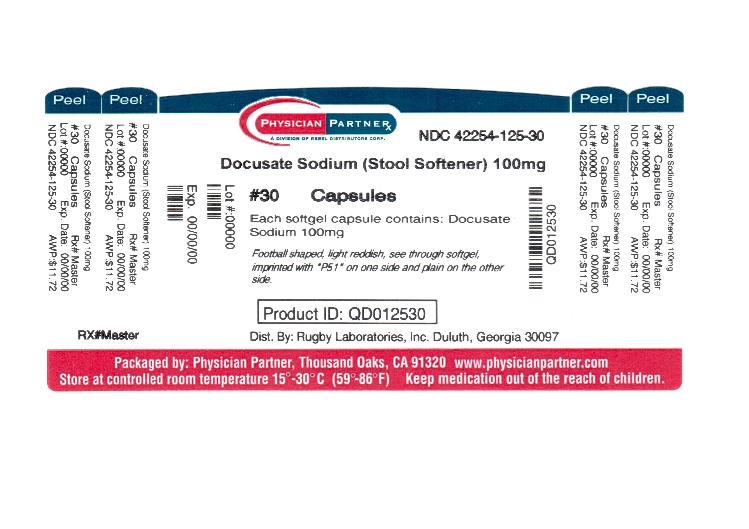
Label: NON-HABIT FORMING STOOL SOFTENER- docusate sodium capsule, liquid filled
-
Contains inactivated NDC Code(s)
NDC Code(s): 42254-125-00, 42254-125-30, 42254-125-60 - Packager: Rebel Distributors Corp
- This is a repackaged label.
- Source NDC Code(s): 0536-3756
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 17, 2012
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient (in each softgel capsule)
- Uses
-
Warnings - Do not use
- for longer than one week
- if you are taking mineral oil
- when abdominal pain, nausea, or vomiting are present
Ask a doctor before use if
you have a sudden change in bowel habits that lasts over two weeks
Ask a doctor or pharmacist before use if you are taking any other drug. Take this product two or more hours before or after other drugs. Laxatives may affect how other drugs work.
- Directions
- Other information
- Inactive Ingredients
- Questions or comments?
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
NON-HABIT FORMING STOOL SOFTENER
docusate sodium capsule, liquid filledProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:42254-125(NDC:0536-3756) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOCUSATE SODIUM (UNII: F05Q2T2JA0) (DOCUSATE - UNII:M7P27195AG) DOCUSATE SODIUM 100 mg Inactive Ingredients Ingredient Name Strength FD&C RED NO. 40 (UNII: WZB9127XOA) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) GELATIN (UNII: 2G86QN327L) GLYCERIN (UNII: PDC6A3C0OX) POLYETHYLENE GLYCOLS (UNII: 3WJQ0SDW1A) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SORBITOL (UNII: 506T60A25R) Product Characteristics Color orange (orange) Score no score Shape OVAL (OVAL) Size 13mm Flavor Imprint Code P51 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:42254-125-30 30 in 1 BOTTLE 2 NDC:42254-125-60 60 in 1 BOTTLE 3 NDC:42254-125-00 100 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part334 03/29/2012 Labeler - Rebel Distributors Corp (118802834) Registrant - PSS World Medical, Inc. (101822862) Establishment Name Address ID/FEI Business Operations PSS World Medical, Inc. 791528623 REPACK(42254-125) Establishment Name Address ID/FEI Business Operations STAT RX USA LLC 786036330 REPACK(42254-125) Establishment Name Address ID/FEI Business Operations Dispensing Solutions, Inc. 066070785 RELABEL(42254-125) , REPACK(42254-125) Establishment Name Address ID/FEI Business Operations SCRIPT PAK 964420108 RELABEL(42254-125) , REPACK(42254-125) Establishment Name Address ID/FEI Business Operations Keltman Pharmaceuticals, Inc. 362861077 REPACK(42254-125) Establishment Name Address ID/FEI Business Operations Rebel Distirbutors Corp. 118802834 RELABEL(42254-125) , REPACK(42254-125)