SORILUX- calcipotriene aerosol, foam
Stiefel Laboratories Inc
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use SORILUX safely and effectively. See full prescribing information for SORILUX.
SORILUX (calcipotriene) foam, 0.005%, for topical use Initial U.S. Approval: 1993 INDICATIONS AND USAGESORILUX™ Foam is a vitamin D analog indicated for the topical treatment of plaque psoriasis of the scalp and body in patients 18 years and older. (1) (1) DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONSAdverse reactions reported in ≥1% of subjects treated with SORILUX Foam and at a higher incidence than subjects treated with vehicle were application site erythema and application site pain. (6.1) (6) To report SUSPECTED ADVERSE REACTIONS, contact Stiefel Laboratories, Inc. at 1-888-784-3335 (1-888-STIEFEL) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. (6) See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling. Revised: 9/2013 |
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
SORILUX Foam is indicated for the topical treatment of plaque psoriasis of the scalp and body in patients 18 years and older.
2 DOSAGE AND ADMINISTRATION
SORILUX Foam is for topical use only. SORILUX Foam is not for oral, ophthalmic, or intravaginal use.
Apply a thin layer of SORILUX Foam twice daily to the affected areas and rub in gently and completely. Avoid contact with the face and eyes.
5 WARNINGS AND PRECAUTIONS
5.1 Flammability
The propellant in SORILUX Foam is flammable. Instruct the patient to avoid fire, flame, and smoking during and immediately following application.
5.2 Effects on Calcium Metabolism
Transient, rapidly reversible elevation of serum calcium has occurred with use of calcipotriene. If elevation in serum calcium outside the normal range should occur, discontinue treatment until normal calcium levels are restored.
5.3 Risk of Ultraviolet Light Exposure
Instruct the patient to avoid excessive exposure of the treated areas to either natural or artificial sunlight, including tanning booths and sun lamps. Physicians may wish to limit or avoid use of phototherapy in patients who use SORILUX Foam. [See Nonclinical Toxicology (13.1).]
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
SORILUX Foam was studied in four vehicle-controlled trials. A total of 1094 subjects with plaque psoriasis, including 654 exposed to SORILUX Foam, were treated twice daily for 8 weeks.
Adverse reactions reported in ≥1% of subjects treated with SORILUX Foam and at a higher incidence than subjects treated with vehicle were application site erythema (2%) and application site pain (3%). The incidence of these adverse reactions was similar between the body and scalp.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Teratogenic Effects, Pregnancy Category C:
There are no adequate and well-controlled trials in pregnant women. Therefore, SORILUX Foam should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Studies of teratogenicity were done by the oral route where bioavailability is expected to be approximately 40-60% of the administered dose. Increased rabbit maternal and fetal toxicity was noted at 12 mcg/kg/day (132 mcg/m2/day). Rabbits administered 36 mcg/kg/day (396 mcg/m2/day) resulted in fetuses with a significant increase in the incidences of incomplete ossification of pubic bones and forelimb phalanges. In a rat study, doses of 54 mcg/kg/day (318 mcg/m2/day) resulted in a significantly higher incidence of skeletal abnormalities consisting primarily of enlarged fontanelles and extra ribs. The enlarged fontanelles are most likely due to calcipotriene's effect upon calcium metabolism. The maternal and fetal no-effect exposures in the rat (43.2 mcg/m2/day) and rabbit (17.6 mcg/m2/day) studies are approximately equal to the expected human systemic exposure level (18.5 mcg/m2/day) from dermal application.
8.3 Nursing Mothers
It is not known whether calcipotriene is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when SORILUX Foam is administered to a nursing woman.
8.4 Pediatric Use
Safety and effectiveness of SORILUX Foam in pediatric patients less than 18 years of age have not been established.
8.5 Geriatric Use
Clinical trials of SORILUX Foam did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients.
10 OVERDOSAGE
Topically applied calcipotriene can be absorbed in sufficient amounts to produce systemic effects. Elevated serum calcium has been observed with use of topical calcipotriene [See Warnings and Precautions (5.2).]
11 DESCRIPTION
SORILUX Foam contains the compound calcipotriene, a synthetic vitamin D3 analog.
Chemically, calcipotriene is (5Z,7E,22E,24S)-24-cyclopropyl-9,10-secochola-5,7,10(19), 22-tetraene-1α,3β,24-triol. The structural formula is represented below:
Molecular Formula: C27H40O3 Molecular Weight: 412.6
Calcipotriene is a white or off-white crystalline substance. SORILUX Foam contains calcipotriene 50 mcg/g in an aqueous-based emulsion foam vehicle consisting of cetyl alcohol, dibasic sodium phosphate, dl-α-tocopherol, edetate disodium, isopropyl myristate, light mineral oil, polyoxyl 20 cetostearyl ether, propylene glycol, purified water, stearyl alcohol, and white petrolatum. SORILUX Foam is dispensed from an aluminum can pressurized with a hydrocarbon (propane/n-butane/isobutane) propellant.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Calcipotriene is a synthetic vitamin D3 analog that has a similar receptor binding affinity as natural vitamin D3. However, the exact mechanism of action contributing to the clinical efficacy in the treatment of psoriasis is unknown.
12.3 Pharmacokinetics
The systemic absorption of calcipotriene in subjects with psoriasis of the body was evaluated at steady state following application of either SORILUX Foam or calcipotriene ointment to a body surface area of 5% to 10%. In the SORILUX Foam treatment group, 15 out of 16 subjects had calcipotriene plasma concentrations below the limit of quantitation (10 pg/mL), while in the calcipotriene ointment treated group, 5 out of 16 subjects had measurable calcipotriene plasma concentrations at various time points. All measurable plasma calcipotriene concentrations were below 25 pg/mL.
The systemic disposition of calcipotriene is expected to be similar to that of the naturally occurring vitamin D. Absorbed calcipotriene is known to be converted to inactive metabolites within 24 hours of application and the metabolism occurs via a similar pathway to the natural hormone.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis: Calcipotriene topically administered to mice for up to 24 months at dose levels of 3, 10, or 30 mcg /kg/day (corresponding to 9, 30, or 90 mcg /m2/day) showed no significant changes in tumor incidence when compared with controls. In a study in which albino hairless mice were exposed to both UVR and topically applied calcipotriene, a reduction in the time required for UVR to induce the formation of skin tumors was observed (statistically significant in males only), suggesting that calcipotriene may enhance the effect of UVR to induce skin tumors [See Warnings and Precautions (5.3).]
Mutagenesis: The genotoxic potential of calcipotriene was evaluated in an Ames assay, a mouse lymphoma TK locus assay, a human lymphocyte chromosome aberration assay, and a mouse micronucleus assay. All assay results were negative.
Impairment of Fertility: Studies in rats at doses up to 54 mcg /kg/day (318 mcg /m2/day) of calcipotriene indicated no impairment of fertility or general reproductive performance.
14 CLINICAL STUDIES
In two multi-center, randomized, double-blind, vehicle-controlled clinical trials a total of 659 subjects with psoriasis were randomized 2:1 to SORILUX Foam or vehicle; subjects applied the assigned treatment twice daily for 8 weeks. Baseline disease severity was graded using a 5-point Investigator Static Global Assessment scale (ISGA), on which subjects scored either “mild” or “moderate” as shown in Table 1.
|
Disease Severity |
Grade |
Definition |
|
Clear |
0 |
No evidence of scaling, erythema, or plaque thickness |
|
Almost clear |
1 |
Occasional fine scale, faint erythema, and barely perceptible plaque thickness |
|
Mild |
2 |
Fine scale with light coloration and mild plaque elevation |
|
Moderate |
3 |
Coarse scale with moderate red coloration and moderate plaque thickness |
|
Severe |
4 |
Thick tenacious scale with deep coloration and severe plaque thickness |
Efficacy evaluation was carried out at Week 8 with treatment success being defined as a score of “clear” (grade 0) or “almost clear” (grade 1) and at least 2 grade improvement from the baseline score. Approximately 30% of enrolled subjects were graded as “mild” on the ISGA scale. The study population ranged in age from 12 to 89 years with 10 subjects less than 18 years of age at baseline. The subjects were 54% male and 88% Caucasian. Table 2 presents the efficacy results for each trial.
|
|
Trial 1 |
Trial 2 |
||
|
|
SORILUX Foam N = 223 |
Vehicle Foam N = 113 |
SORILUX Foam N = 214 |
Vehicle Foam N = 109 |
|
Number (%) of Subjects with Treatment Success |
31 (14%) |
8 (7%) |
58 (27%) |
17 (16%) |
In one trial, subjects graded as “mild” at baseline showed a greater response to vehicle than SORILUX Foam.
Table 3 presents the success rates by disease severity at baseline for each trial.
|
Trial 1 |
Trial 2 |
|||
|
ISGA Scores at Baseline |
SORILUX Foam (N = 223) |
Vehicle Foam (N = 113) |
SORILUX Foam (N = 214) |
Vehicle Foam (N = 109) |
|
Mild |
2/73 (2.7%) |
3/34 (8.8%) |
8/56 (14.3%) |
4/31 (12.9%) |
|
Moderate |
29/150 (19.3%) |
5/79 (6.3%) |
50/158 (31.6%) |
13/78 (16.7%) |
In another multi-center, randomized, double-blind, vehicle-controlled clinical trial, a total of 363 subjects with moderate plaque psoriasis of the scalp and body were randomized 1:1 to SORILUX Foam or vehicle. Subjects applied the assigned treatment to the affected areas twice daily for 8 weeks. Baseline disease severity of the scalp was graded using a 6-point ISGA; a score of "moderate" corresponded to grade 3.
The primary efficacy evaluation for scalp involvement was carried out at Week 8 with treatment success being defined as a score of “clear” (grade 0) or “almost clear” (grade 1). The study population ranged in age from 12 to 97 years with 11 subjects less than 18 years of age at baseline. The subjects were 60% male and 87% Caucasian. Table 4 presents the efficacy results for the trial.
|
Trial 3 |
||
|
SORILUX Foam N = 181 |
Vehicle Foam N = 182 |
|
|
Number (%) of Subjects with Treatment Success |
74 (41%) |
44 (24%) |
The contribution to efficacy of individual components of the vehicle has not been established.
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling (Patient Information)
Inform the patient to adhere to the following instructions:
- •
- Avoid excessive exposure of the treated areas to either natural or artificial sunlight, including tanning beds and sun lamps.
- •
- Avoid contact with the face and eyes. If SORILUX Foam gets on the face or in or near their eyes, rinse thoroughly with water.
- •
- Apply SORILUX Foam to the scalp when the hair is dry.
- •
- Talk to your doctor if your skin does not improve after treatment with SORILUX Foam for 8 weeks.
- •
- Wash your hands after applying SORILUX Foam unless your hands are the affected site.
- •
- Avoid fire, flame, and smoking during and immediately following application since SORILUX Foam is flammable.
- •
- Do not place SORILUX Foam in the refrigerator or freezer.
SORILUX is a trademark of Stiefel Laboratories, Inc.
Manufactured for
Stiefel Laboratories, Inc.
Research Triangle Park, NC 27709
©2013, Stiefel Laboratories, Inc.
SOR:6PI
|
PHARMACIST—DETACH HERE AND GIVE INSTRUCTIONS TO PATIENT |
_ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _
Patient Information
SORILUX (SOR-i-lux)
(calcipotriene)
Foam
Important: For skin use only. Do not get SORILUX Foam on your face or in your eyes, mouth, or vagina.
Read the Patient Information before you start using SORILUX Foam and each time you get a refill. There may be new information. This information does not take the place of talking with your doctor about your medical condition or treatment.
What is SORILUX Foam?
SORILUX Foam is a prescription medicine used on the skin (topical) to treat plaque psoriasis of the scalp and body in people 18 years and older.
It is not known if SORILUX Foam is safe and effective in people under 18 years old.
Who should not use SORILUX Foam?
Do not use SORILUX Foam if you have been told by your doctor that you have a high level of calcium in your blood (hypercalcemia).
What should I tell my doctor before using SORILUX Foam?
Before you use SORILUX Foam, tell your doctor if you:
- •
- are getting light therapy for your psoriasis
- •
- have any other medical conditions
- •
- are pregnant or planning to become pregnant. It is not known if SORILUX Foam can harm your unborn baby. Talk to your doctor if you are pregnant or plan to become pregnant.
- •
- are breastfeeding. It is not known if SORILUX Foam passes into breast milk. Do not apply SORILUX Foam to the chest area if you are breastfeeding a baby. This will help to prevent the baby from accidentally getting SORILUX Foam into their mouth.
Tell your doctor about all the medicines you take, including prescription and nonprescription medicines, vitamins, and herbal supplements.
Know the medicines you take. Keep a list of your medicines with you to show your doctor and pharmacist when you get a new medicine.
How should I use SORILUX Foam?
- •
- Apply SORILUX Foam exactly as prescribed. SORILUX Foam is usually applied to the affected skin areas two times each day.
- •
- SORILUX Foam is for use on the skin only. Do not get SORILUX Foam in your eyes, mouth, or vagina.
- •
- SORILUX Foam is flammable. Avoid fire, flame, or smoking during and right after you apply SORILUX Foam to your skin.
- •
- Avoid excessive natural or artificial sunlight including tanning booths and sunlamps. Wear a hat and clothes that cover the treated areas of your skin if you have to be in sunlight.
Instructions for applying SORILUX Foam
1. Before applying SORILUX Foam for the first time, break the tiny plastic piece at the base of the can’s rim by gently pushing back (away from the piece) on the nozzle. See Figure A.
Figure A
2. Shake the can of SORILUX Foam before use. See Figure B.
3. Turn the can of SORILUX Foam upside down and press the nozzle. See Figure C.
Figure C
4. Dispense a small amount of SORILUX Foam into the palm of your hand. See Figure D.
5. Use enough SORILUX Foam to cover the affected area with a thin layer. Apply SORILUX Foam to your scalp when your hair is dry. Part your hair and apply directly on the affected area. Gently rub the foam into the affected area until it disappears into the skin. See Figures E, F and G.
Figure E
Figure F
Figure G
6. Avoid getting SORILUX Foam on your face or in or near the eyes, mouth, or vagina. If SORILUX Foam gets on your face or in or near your eyes, rinse with water. Wash hands after applying SORILUX Foam unless your hands are a treated area.
What are the possible side effects of SORILUX Foam?
The most common side effects of SORILUX Foam are redness and pain of the treated skin areas.
Tell your doctor if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of SORILUX Foam. Ask your doctor or pharmacist for more information.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
You may also report side effects to Stiefel Laboratories, Inc. at 1-888-784-3335.
How should I store SORILUX Foam?
- •
- Store SORILUX Foam at room temperature, between 68°F to 77°F (20C° to 25°C).
- •
- SORILUX Foam is flammable. Keep the can away from all sources of fire and heat.
- •
- Do not spray SORILUX Foam near fire or direct heat. Never throw the can into a fire, even if the can is empty.
- •
- Do not puncture the can of SORILUX Foam.
Keep SORILUX Foam and all medicines out of the reach of children.
General Information about SORILUX Foam
Medicines are sometimes prescribed for purposes other than those listed in Patient Information leaflets. Do not use SORILUX Foam for a condition for which it was not prescribed. Do not give SORILUX Foam to other people even if they have the same symptoms that you have. It may harm them.
This Patient Information leaflet summarizes the most important information about SORILUX Foam. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about SORILUX Foam that is written for health professionals.
What are the ingredients of SORILUX Foam?
Active ingredient: calcipotriene
Inactive ingredients: cetyl alcohol, dibasic sodium phosphate, dl-α-tocopherol, edetate disodium, isopropyl myristate, light mineral oil, polyoxyl 20 cetostearyl ether, propylene glycol, purified water, stearyl alcohol, and white petrolatum. The foam is dispensed from an aluminum can pressurized with a hydrocarbon (propane/n-butane/isobutane) propellant.
This Patient Information has been approved by the U.S. Food and Drug Administration.
SORILUX is a trademark of Stiefel Laboratories, Inc.
Manufactured for:
Stiefel Laboratories, Inc.
Research Triangle Park, NC 27709
©2013, Stiefel Laboratories, Inc
September 2013
SOR:6PIL
| SORILUX
calcipotriene aerosol, foam |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Stiefel Laboratories Inc (808842343) |
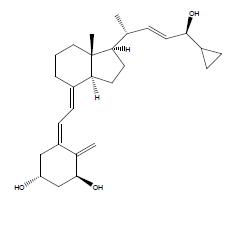

 Figure B
Figure B 
 Figure D
Figure D